Draft Guidance Document: Applications for Medical Device Investigational Testing Authorizations
This draft guidance document reflects Health Canada’s current thinking on Investigational Testing Authorizations (ITA) for medical devices and may be subject to changes as policy develops. The document clarifies application requirements and processes, including pre-ITA meetings, format for an ITA application and filing requests for revisions to an ITA.
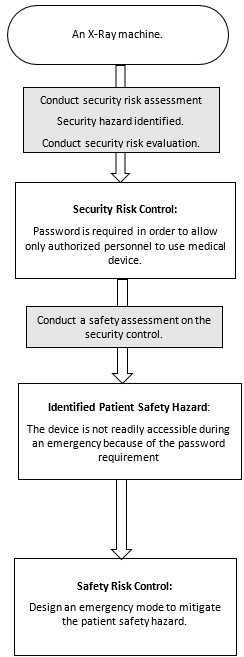
Guidance Document: Pre-market Requirements for Medical Device Cybersecurity

Current Medical Device Regulations in Canada
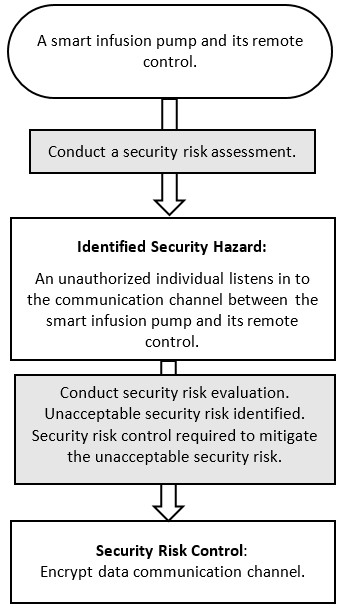
Guidance Document: Pre-market Requirements for Medical Device Cybersecurity
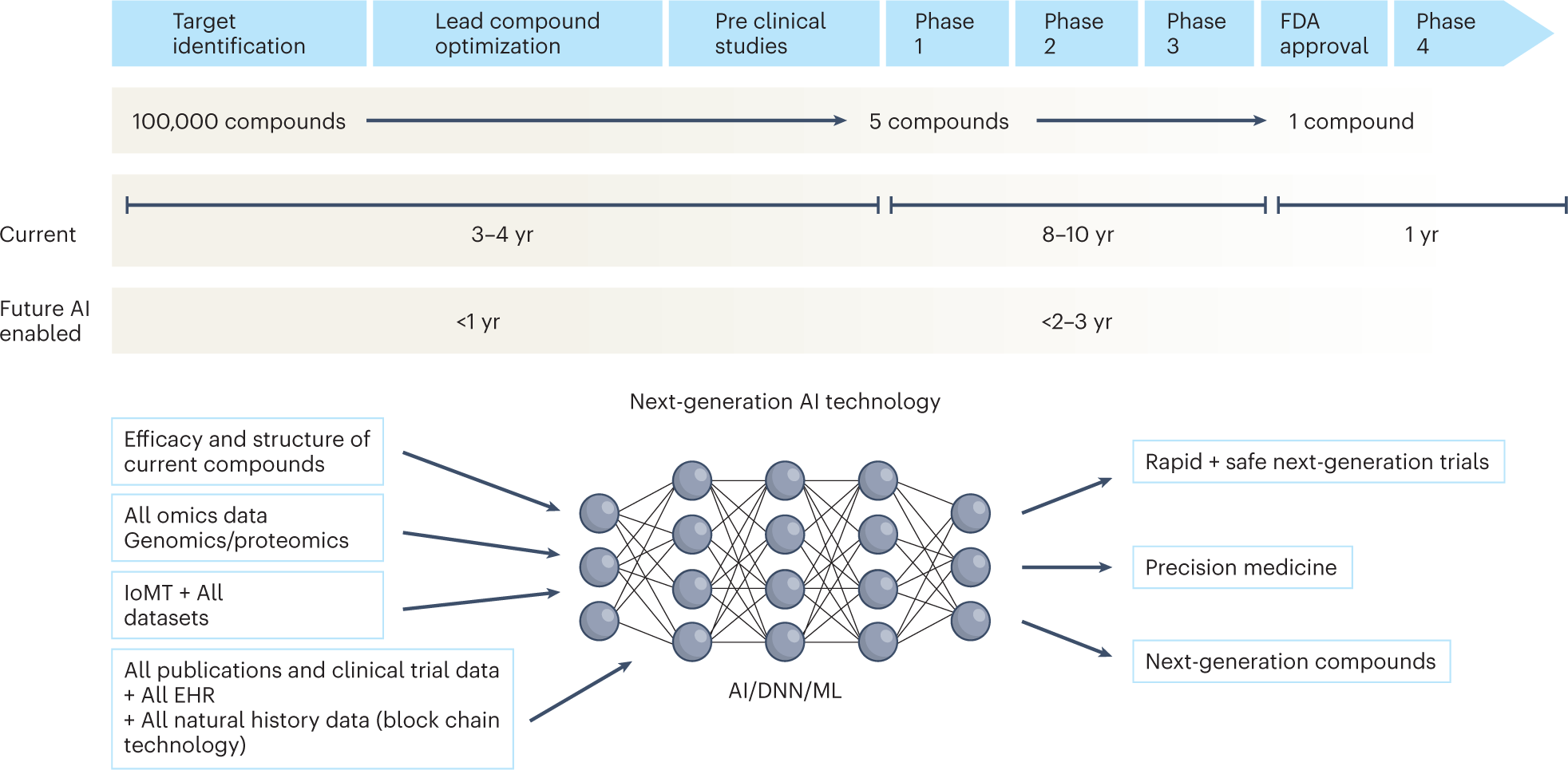
The next generation of evidence-based medicine

Streamlining Postapproval Submissions Using ICH Q12 & SCDM

Applications for Medical Device Investigational Testing Authorizations Guidance Document
.png)
eCRF: Electronic Case Report Form in Clinical Trials - Essential Guide

Guidance Document: Pre-market Requirements for Medical Device Cybersecurity

Class II - IV Medical Device Investigational Testing in Canada - Vantage BioTrials

Quality by design (QbD) approach in marketing authorization procedures of Non-Biological Complex Drugs: A critical evaluation - ScienceDirect
Decentralized Procedure for Marketing Authorization in EU (1-2) 5.