At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
At Critical Temperature,pressure and volume . The compressibility Factor (Z) Is
At Critical Temperature-pressure and volume - The compressibility Factor -Z- Is

At critical temperature, pressure and volume. The compressibility fact

At Critical Temperature,pressure and volume . The compressibility

Math Physics Chemistry Questions Discussion Lists - Dated: 2020-12-02
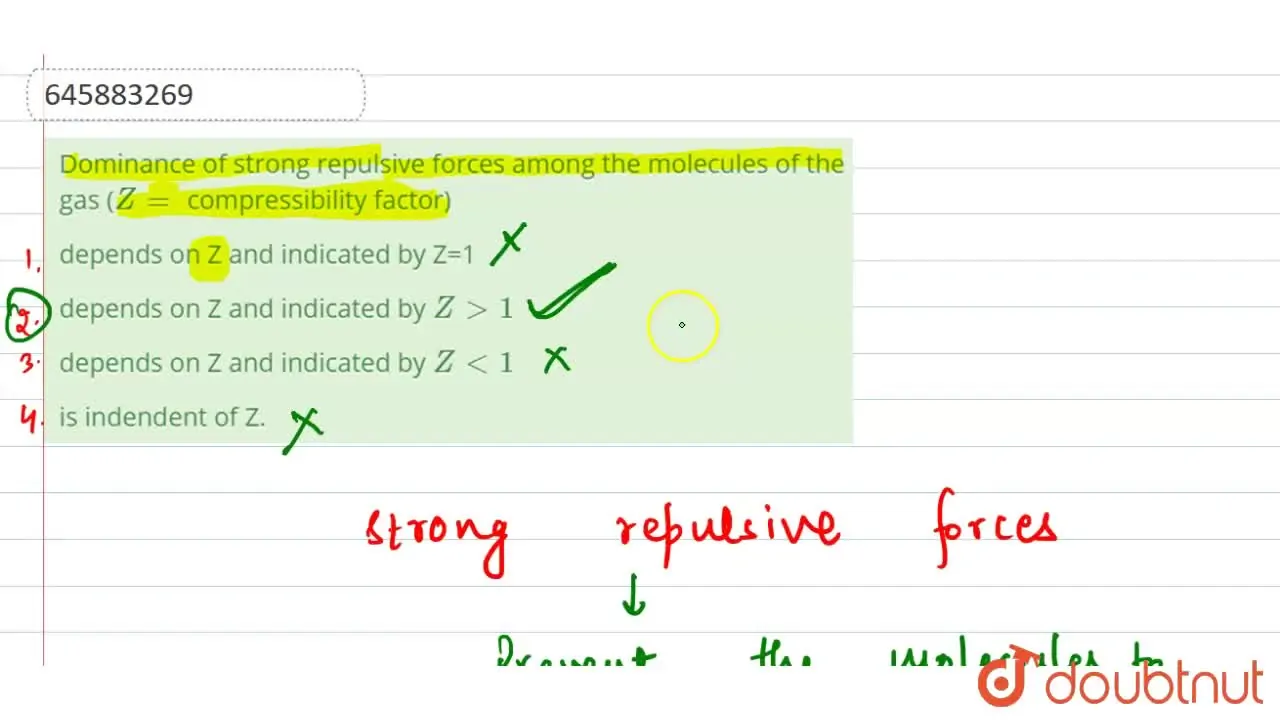
Dominance of strong repulsive forces among the molecules of the gas
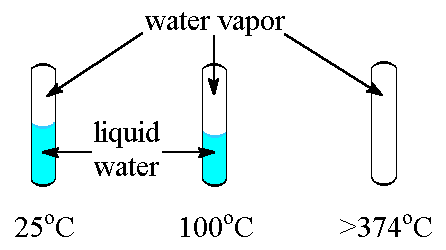
Critical Temperature and Pressure

Math Physics Chemistry Questions Discussion Lists - Dated: 2020-12-02

At critical temperature, pressure and volume. The compressibility factor (Z) is 2

States of Matter, PDF, Gases

At what temperature will be total kinetic energy (KE) of 0.30 mole

Math Physics Chemistry Questions Discussion Lists - Dated: 2020-12-02

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

A plot of Dranchuk Abou Kassem z factor chart with convergence problem

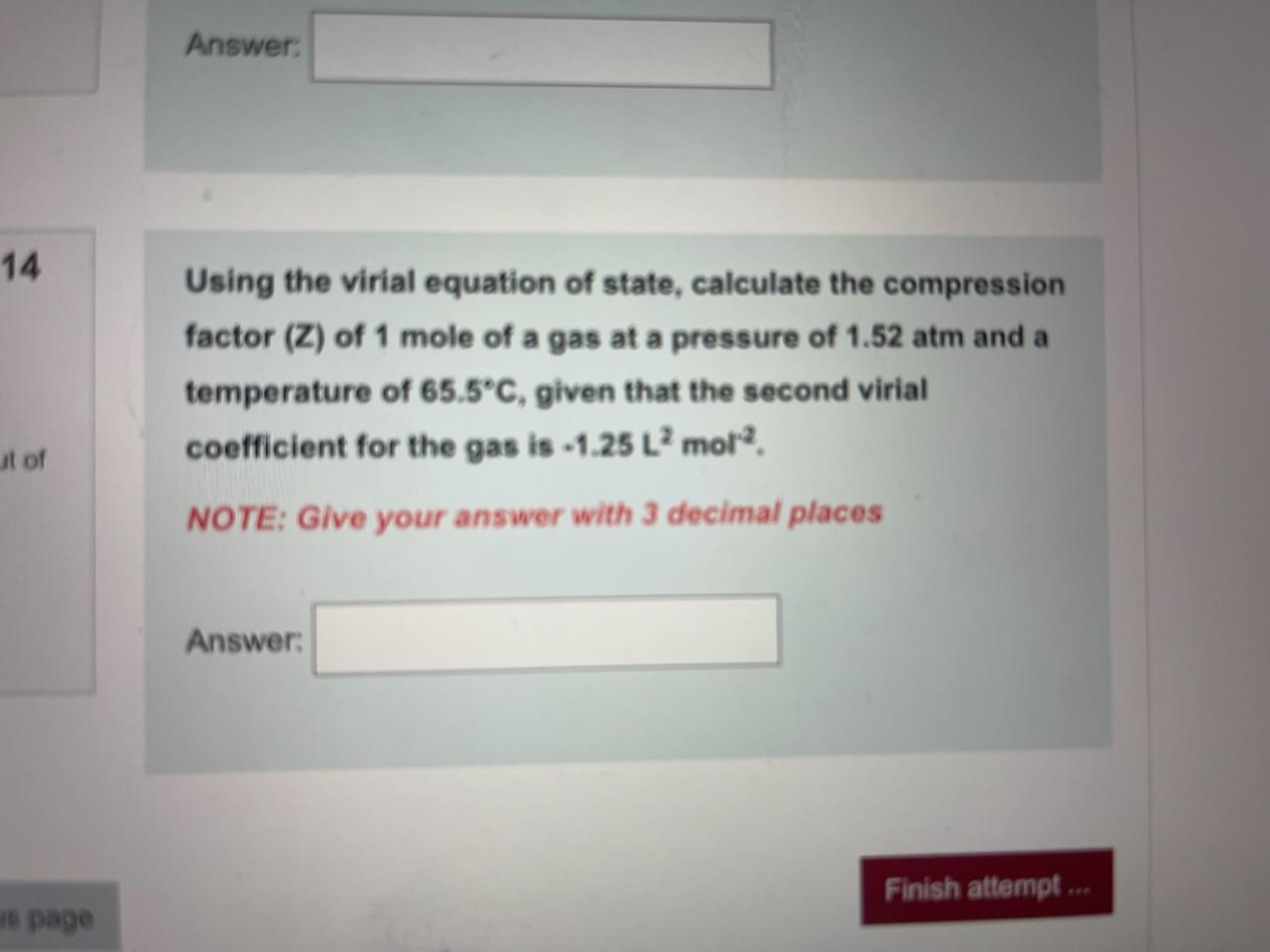
Consider a graph between compressibility factor Z and pressure P

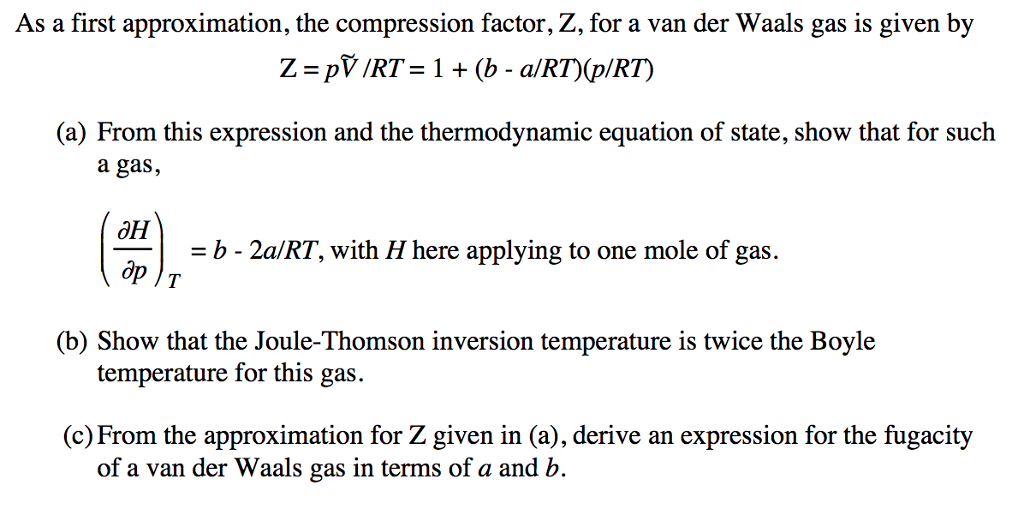
6.3: Van der Waals and Other Gases - Physics LibreTexts

Math Physics Chemistry Questions Discussion Lists - Dated: 2020-12-02



:format(webp)/https://static-hk.zacdn.com/p/under-armour-4521-8650536-1.jpg)


