At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior (JEE MAINS 2019) - Doctor Logics Sunny Garg Chemistry
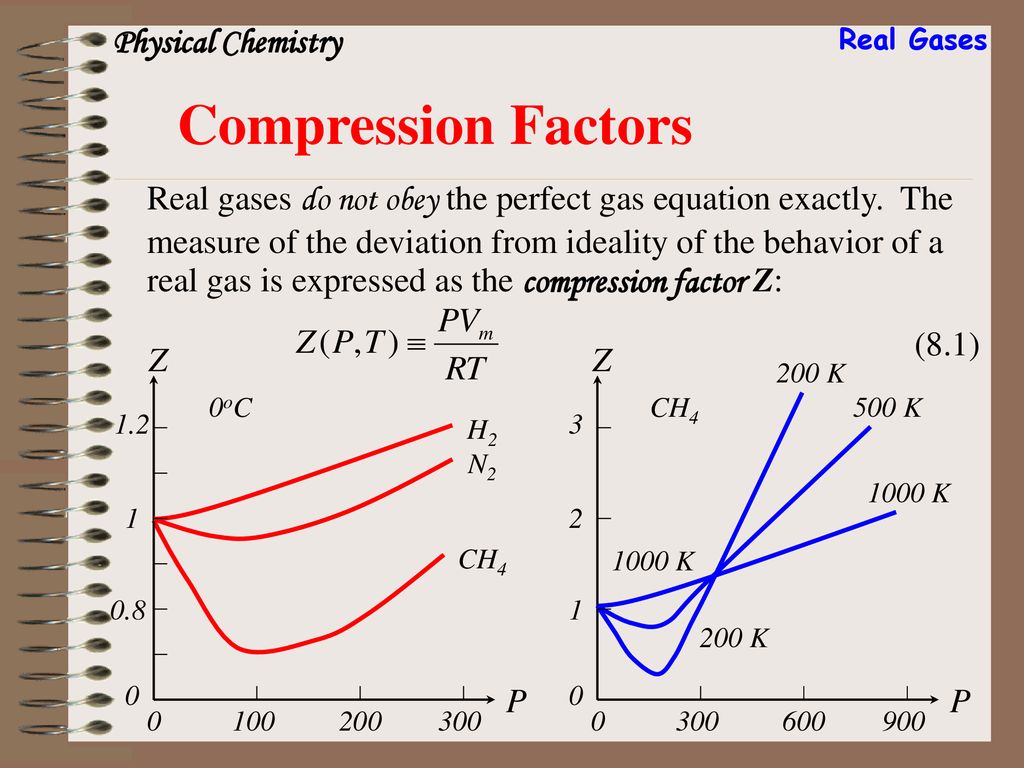
At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behavior. Their equation of state is given as P=RTV−b at T. Here, b is the van der Waals constant. Which gas will exhibit steepest increase in the plot of Z (compression factor) vs P?

14PIN W At a given temperature T. gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour. Their equation of state is given as pek T. Here, is
At a given temperature T, gases Ne, Ar, Xe and Kr are found to deviate from ideal gas behaviour. - Sarthaks eConnect

At a given temperature T gases Ne Ar Xe and Kr are found to deviate from ideal gas behavior. jee

The temperature of an ideal gas is increased from 27∘ C to 127∘ C. Then, percentage increase in V rms isA. 37 %B. 11 %C. 33 %D. 15.5 %

JEE Advanced 2018 Paper 2 Offline, Thermodynamics Question 17, Chemistry
Kinetic Theory of Gases - JEE Main Previous Year Questions with Solutions

At a given temperature T, gases Ne,Ar,Xe and Kr are found to deviate from..
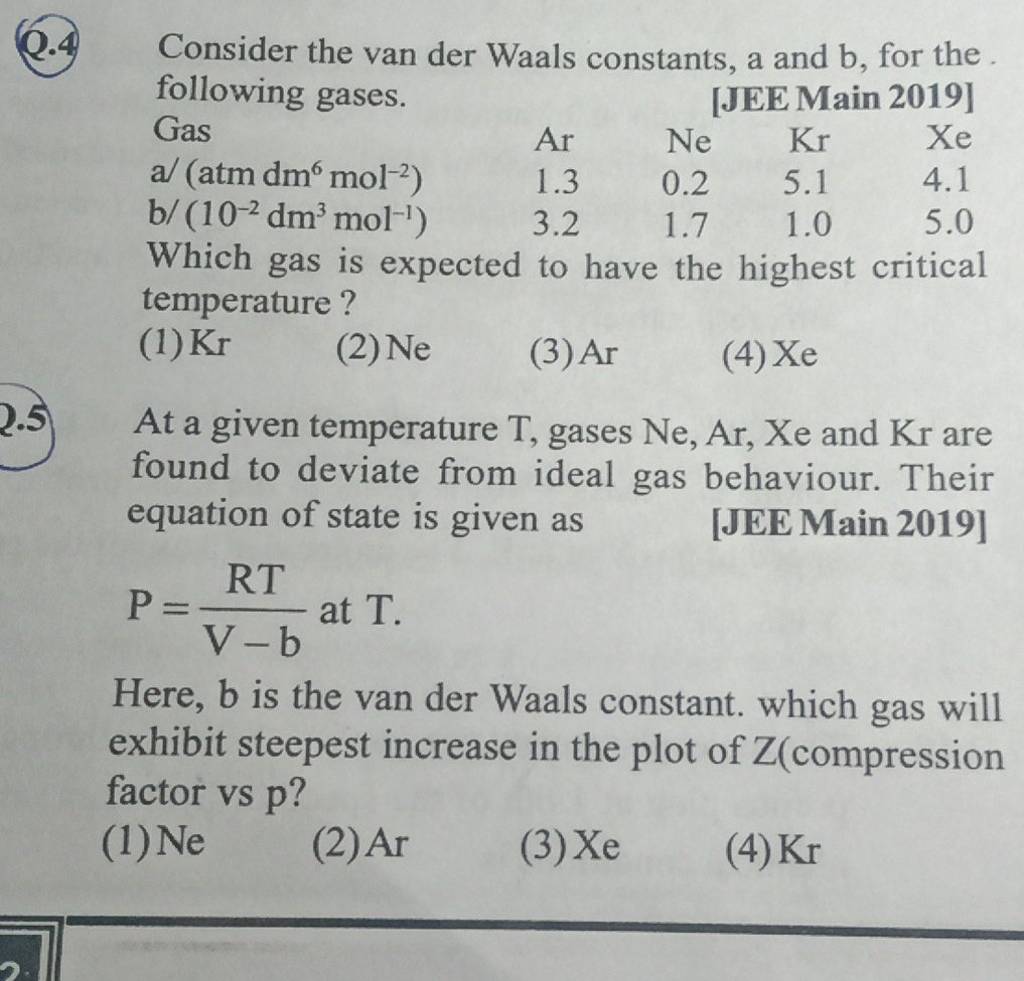
At a given temperature T, gases Ne,Ar,Xe and Kr are found to deviate from..

PDF) Thermal energy storage Diego Armando Gutierrez Diaz

Sol Gel Book, PDF, Solid Oxide Fuel Cell
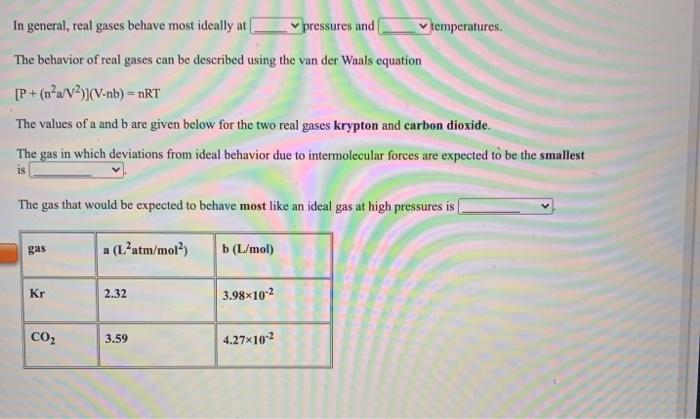
Solved In general, real gases behave most ideally at