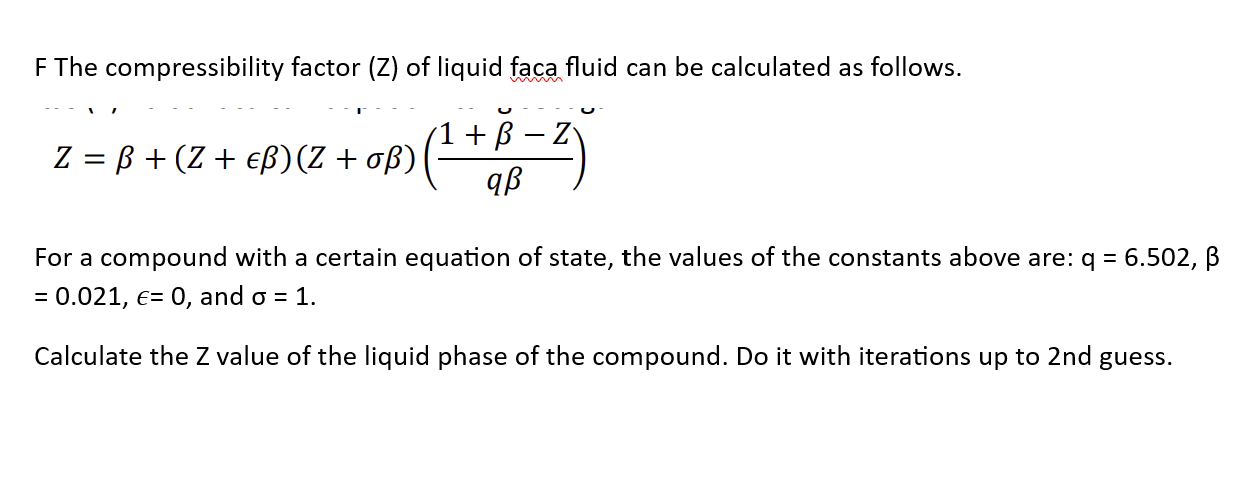
At a high pressure, the compressibility factor (Z) of a real gas is us
At high P. P gt gt (n^(2)a)/(V^(2)) So ‘a’ can be neglected.

Compressibility factor (z): real gases deviate from ideal behav-Turito

Compressibility Factor Calculator

At a high pressure, the compressibility factor (Z) of a real gas is us

The compressiblity factor Z for 1 mole of a real gas at low pressure can be written as

Gas Z Factor Calculator: Dranchuk-Abou-Kassem · PVT Solver

If assertion is true but reason is false.

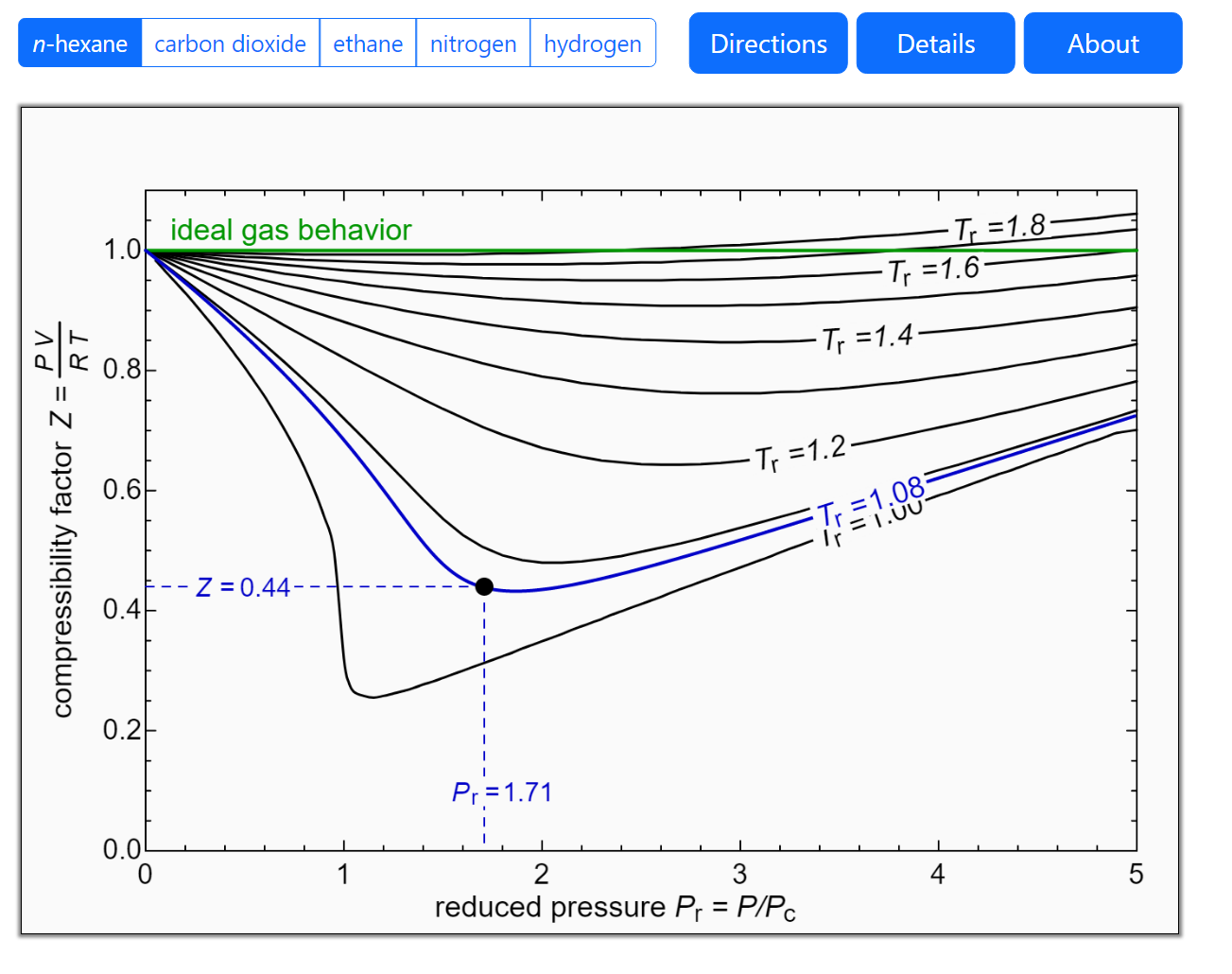
Ch2, Lesson E, Page 9 - Generalized Compressibility Chart

JEE: Van der Waals Equation, Chemistry By Unacademy

A : At high pressure , the compressibility factor Z is (1 + (pb)/(RT))
/wp-content/uploads/2023/05/compress

The role of the compressibility factor Z in describing the volumetric behavior of gases

Gas compressibility factor Z: Ideal gas vs Real gas

At a constant pressure, what should be the percentage increase in the

Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility factor Z - Gaseous State