At high pressure, the compressibility factor 'Z' is equal toa
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to

Gas compressibility factor Z: Ideal gas vs Real gas

Compressibility factor - Wikipedia
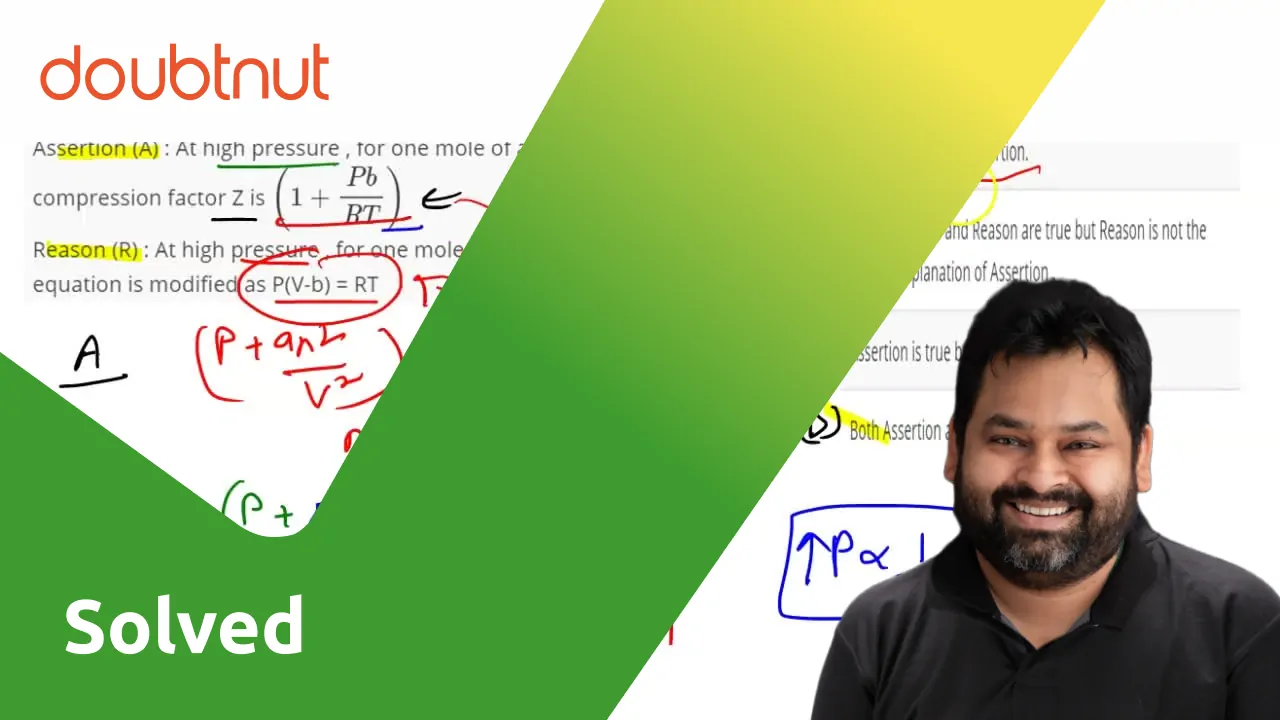
If assertion is true but reason is false.
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

Compressibility factor - Wikipedia
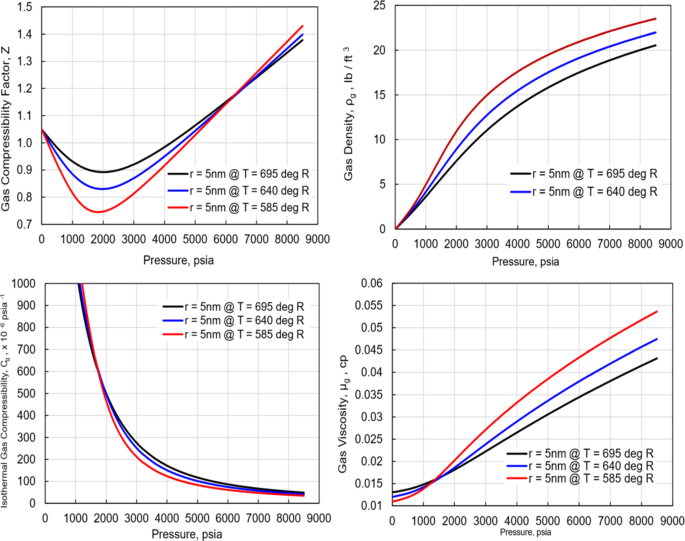
Investigation of the Properties of Hydrocarbon Natural Gases Under Confinement in Tight Reservoirs Due to Critical Properties Shift

NEET Chemistry Chapter Wise Mock Test - Mock Test 2 - CBSE Tuts

The value of compressibility factor at the critical state the gas matches with the `Z_(c )` is
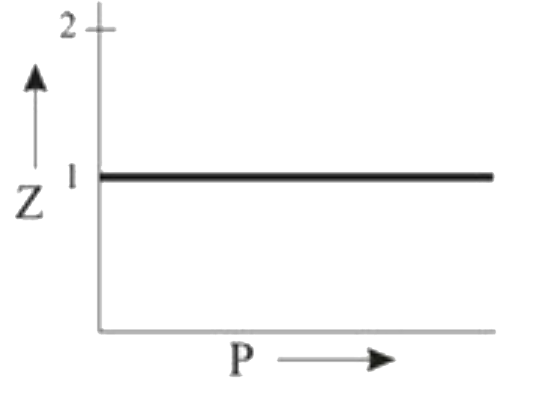
Which of the following represents a plot of compressibility factor (Z)

NEET Chemistry Chapter Wise Mock Test - Mock Test 2 - CBSE Tuts
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

Compressibility Factor Calculator
Solved An ideal gas has a compressibility factor of Z = 1 at