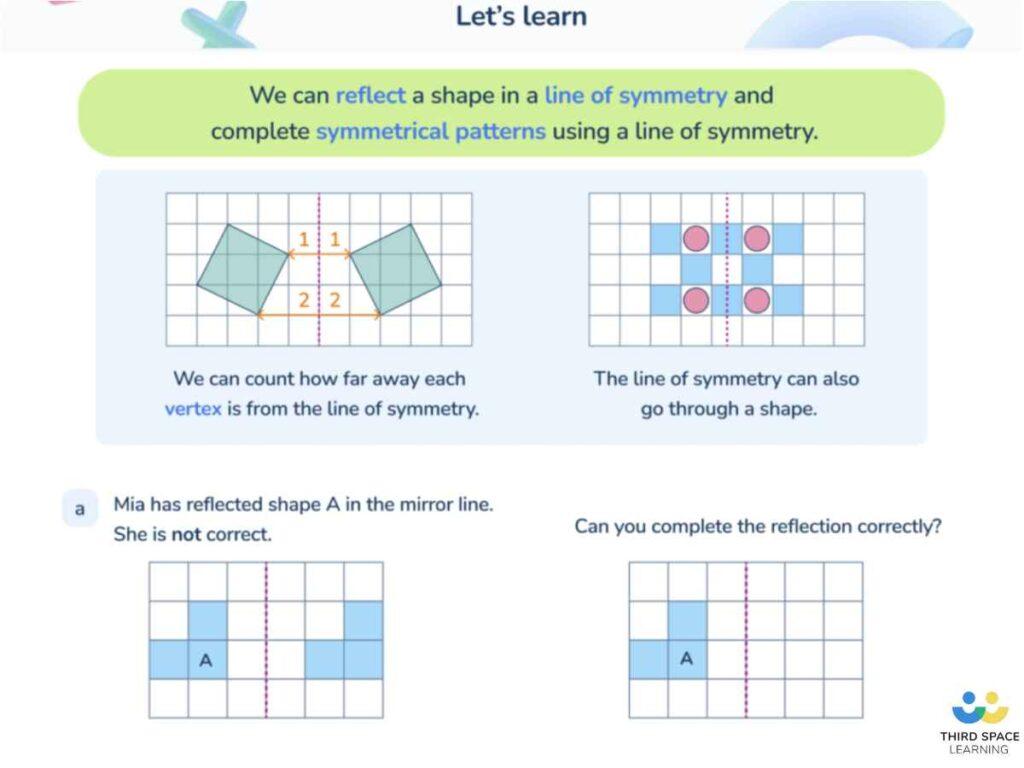
Breaking local symmetry—why water freezes but silica forms a glass
Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water
Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water's cousin, silica, exhibits wayward behavior when cooled that has long puzzled scientists.

Critical cooling rate versus reduced glass transition temperature T rg
Understanding water's anomalies with locally favoured structures

Breaking translational symmetry via polymer chain overcrowding in molecular bottlebrush crystallization

Fast crystal growth of ice VII owing to the decoupling of translational and rotational ordering

Glass transition - Wikipedia

Coatings, Free Full-Text
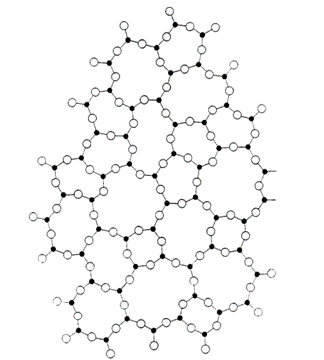
Through looking glass: strange atomic structure of glassy materials
Does water become less dense as it becomes colder or only when it reaches freezing temperature? - Quora

Materials, Free Full-Text

Two ice growth modes on hydrophilic and hydrophobic surfaces. (A) A
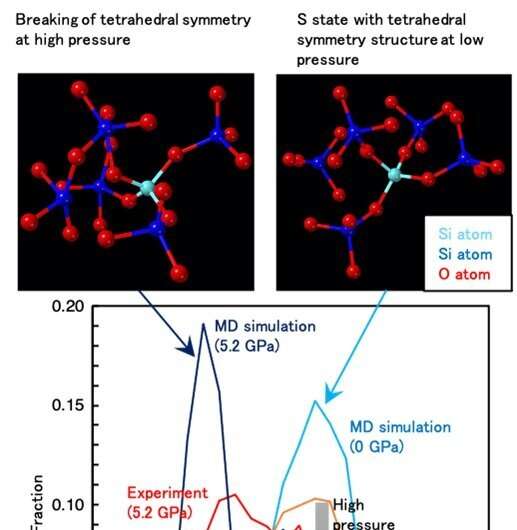
Structural origin of the anomalous properties of SiO2 glass under pressure

Nanoconfined 1D Melting. Different 1D ice structures in zigzag SWCNTs.
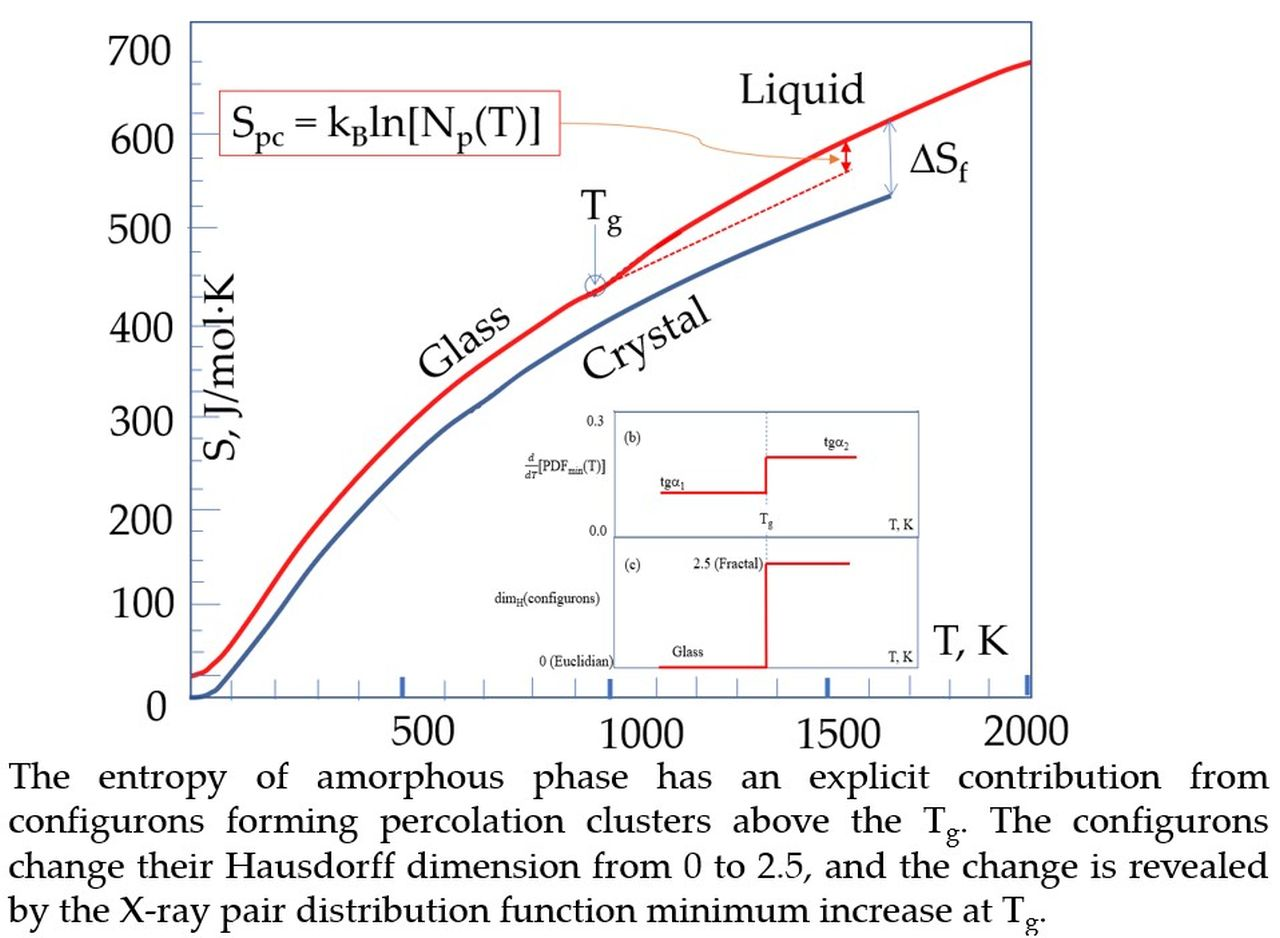
Materials, Free Full-Text