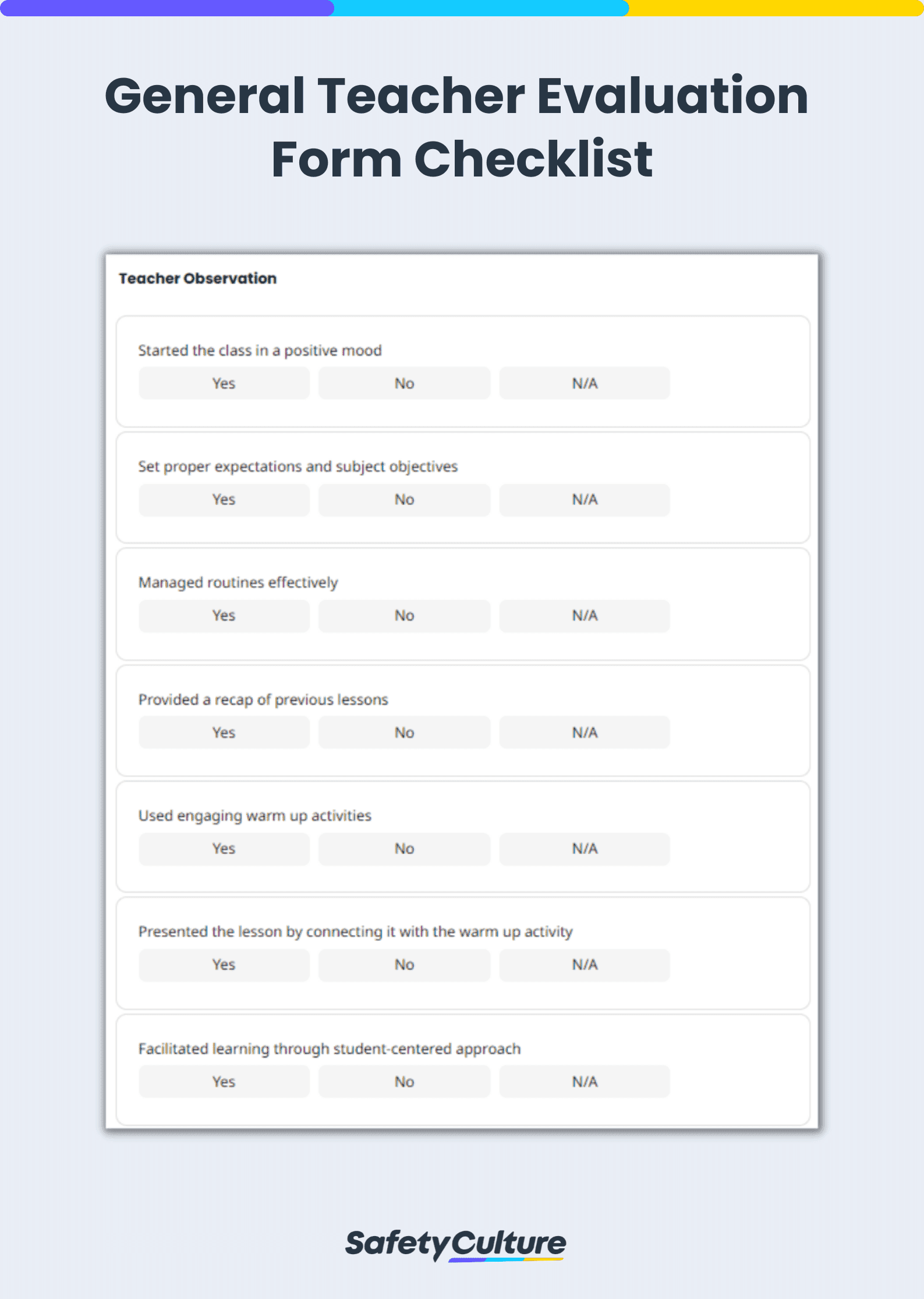
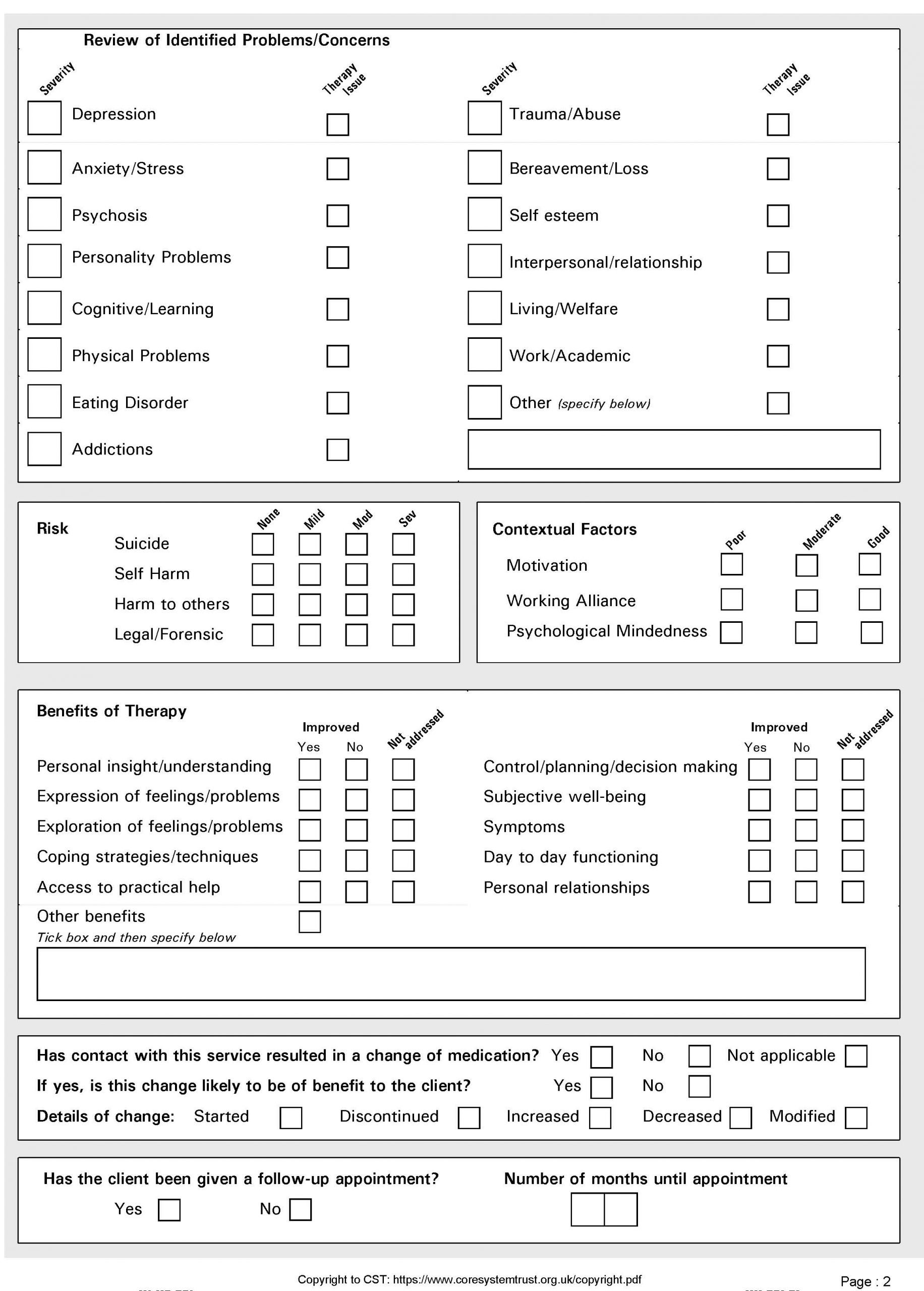
CORE-A End of Therapy (EoT) information : Clinical Outcomes in

Severe Hepatopathy in National Wilms Tumor Studies 3-5: Prevalence, Clinical Features, and Outcomes After Reintroduction of Chemotherapy
FAQ: I need an image for my thesis : Clinical Outcomes in Routine Evaluation (and CST)
/files/Articles/1032531/fmed-10

Patient profile using full CORE-OM (F), CORE Short Form A and B
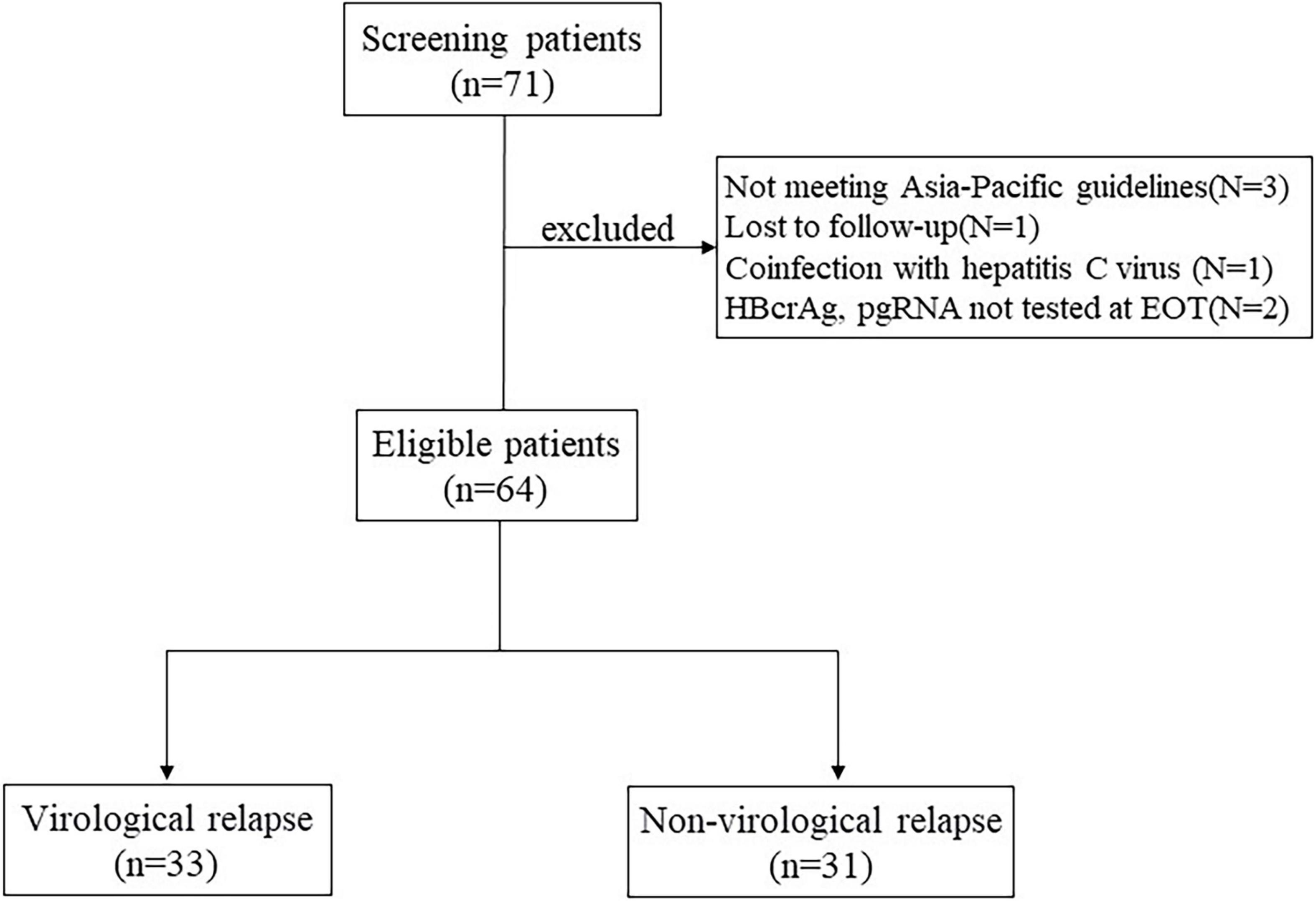
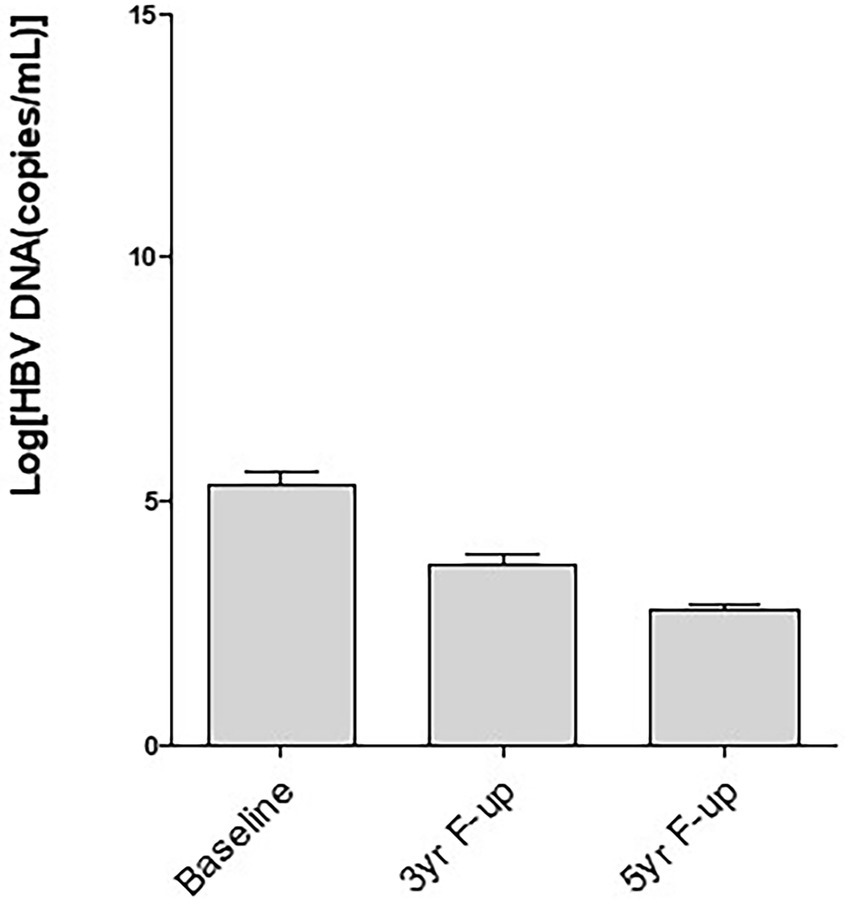
Frontiers Serum Pregenomic RNA Combined With Hepatitis B Core-Related Antigen Helps Predict the Risk of Virological Relapse After Discontinuation of Nucleos(t)ide Analogs in Patients With Chronic Hepatitis B

Talking Therapists: Monitoring Change And Outcomes With The CORE System - WriteUpp Blog

End-of-treatment HBcrAg and HBsAb levels identify durable functional cure after Peg-IFN-based therapy in patients with CHB - ScienceDirect

PDF) A CORE approach to practice-based evidence: A brief history of the origins and applications of the CORE-OM and CORE System

JCM, Free Full-Text

2019 EOT assessment image - Monash Doctors Education

Fillable PDF forms for CORE measures : Clinical Outcomes in Routine Evaluation (and CST)

Trial flow-chart 1 Visit name abbreviations: End of Treatment (EOT)

A, Percentage change from baseline in mEASI score at the EOT (the