
AIROS Medical Receives FDA Clearance to Market New Peristaltic
AIROS Medical announces FDA 510k clearance to market the AIROS 8P compression device and Pants garment that treats leg and pelvic swelling.

Compression Wear And Shapewear Market Trend Analysis, And Forecast To 2033

Airlife 208 - McKesson Medical-Surgical

AIROS Medical Receives FDA Clearance to Market New Peristaltic Pneumatic Compression Device, Truncal Garments for Lymphedema Treatment - AIROS Medical, Inc.
Airsupra (albuterol and budesonide) FDA Approval History

MyChesCo's Regional News Southeastern Pennsylvania News- Page 456 of 563

AIROS Medical Launches AIROS 6P Pneumatic Compression Device and Truncal Garment System - AIROS Medical, Inc.

New AVANOS MEDICAL 7180-20 MIC Safety Percutaneous Endoscopic Gastrostomy (PEG) Kit , 20Fr , Type: Pull (Expired) Disposables - General For Sale - DOTmed Listing #4618512

Innovative Wound Solutions
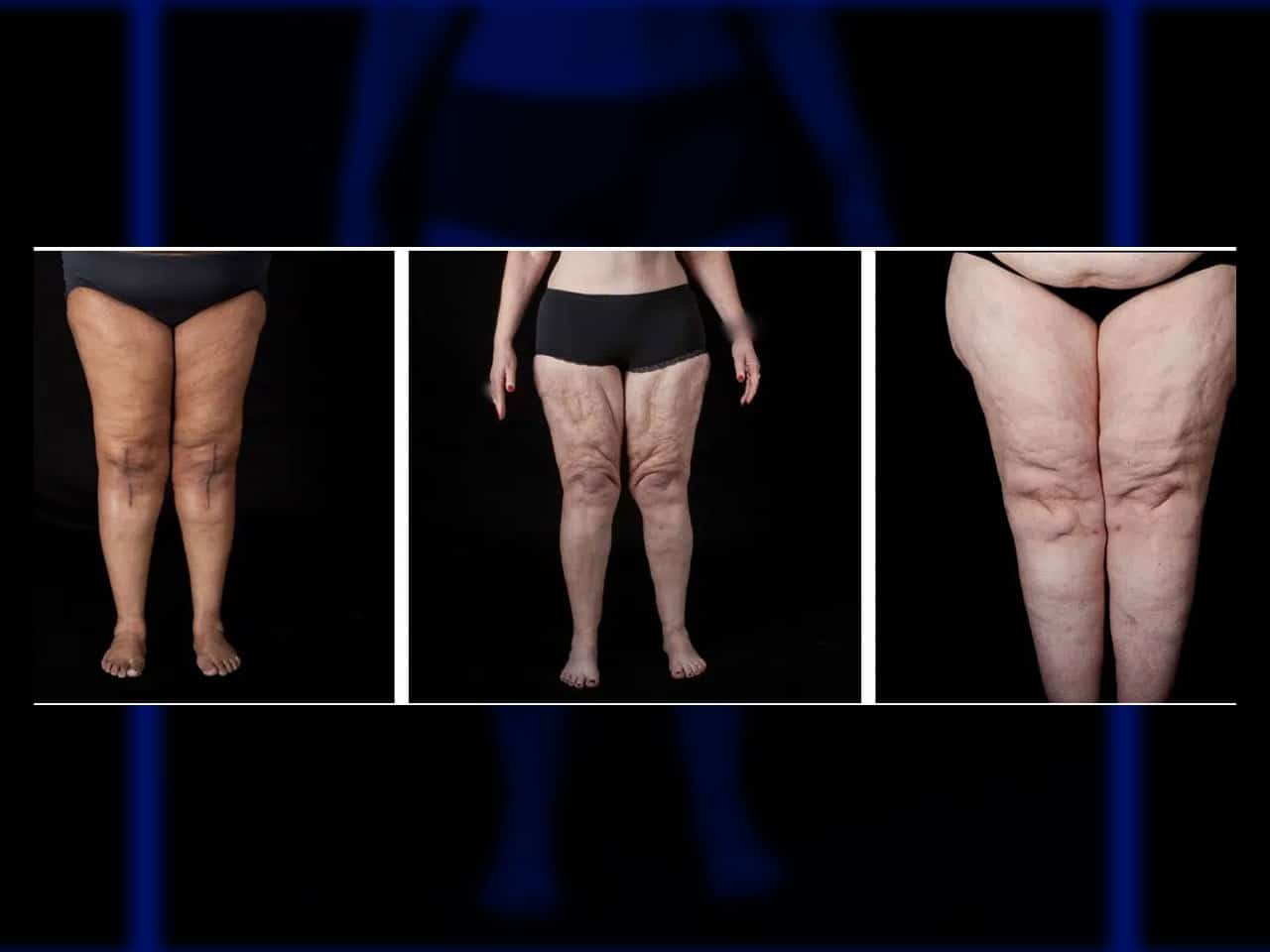
Articles & News - Page 2 of 7 - AIROS Medical, Inc.

FDA approves Beyond Air LungFit PH to treat hypoxic respiratory failure

Navy Removal Scout 800 Pink Pill Assasin Expo Van Travel Bothell Punishment Shred Norelco District Ditch Required Anyhow, PDF

Air Relax AR-1 Professional Compression Boots, Leg









