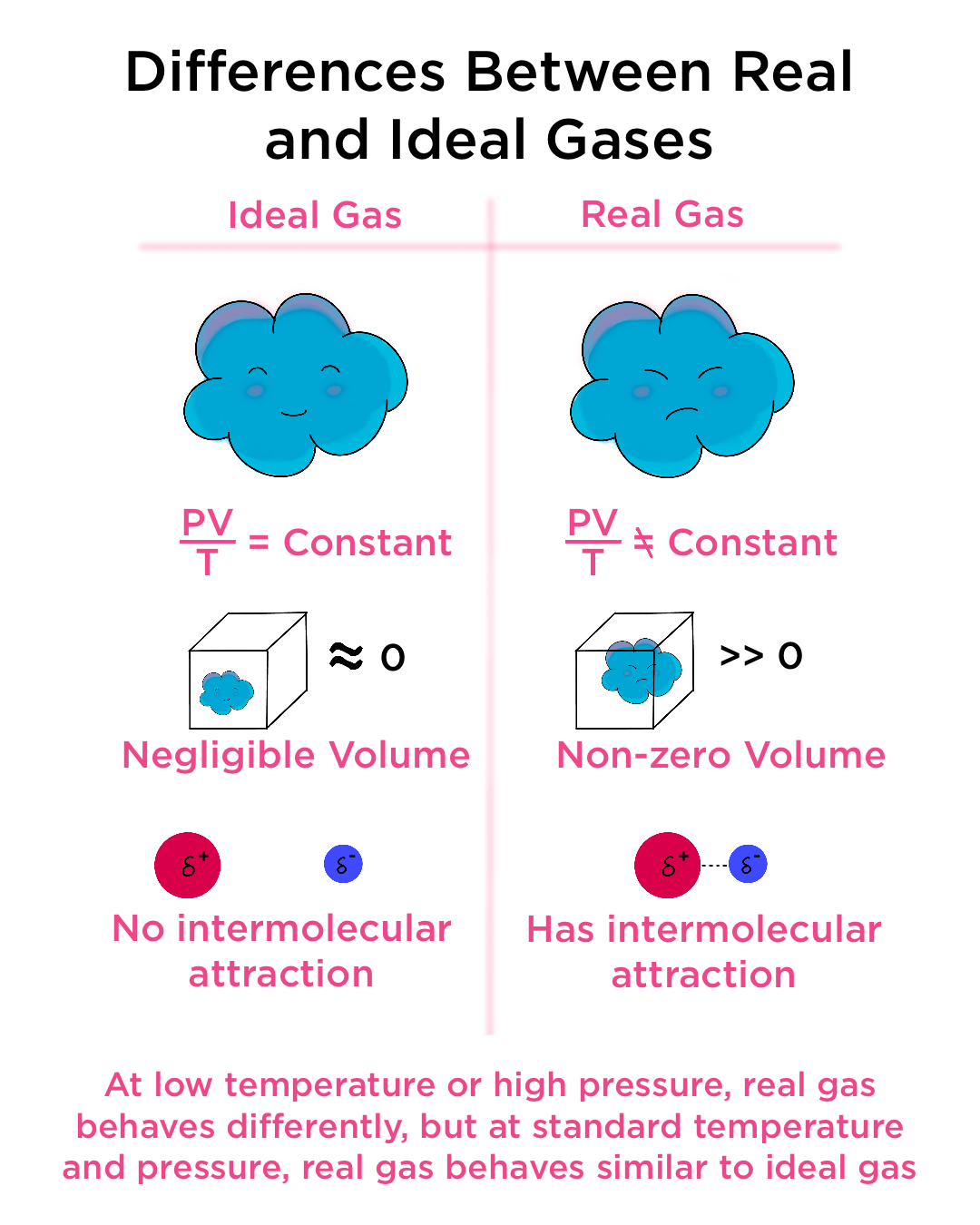
Ideal Gas Assumptions - Kinetic Theory
When considering a gas as an ideal gas and applying the ideal gas law pV=nRT, we need to make 4 assumptions. (1) The volume of a molecule within the gas is n
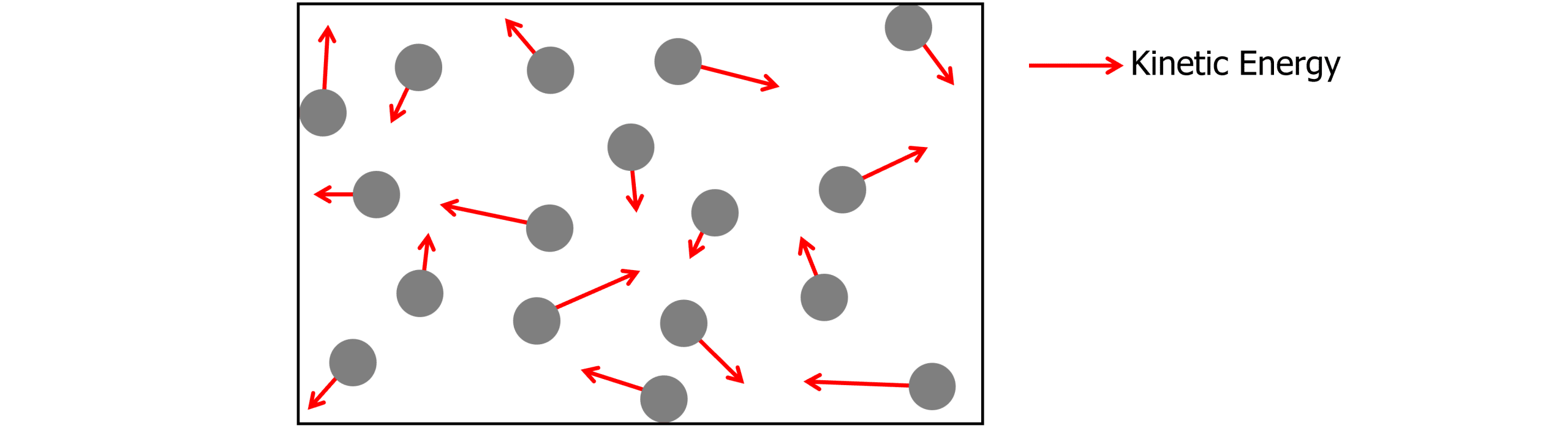
Molecular Kinetic Theory Model Worksheets, Questions and Revision

SOLUTION: Kinetic theory of gases - Studypool

Kinetic Theory of Gases Formula, Assumptions & History - Physics
What is kinetic theory? - Quora

When does a real gas show behaviour same as ideal P gas: W (1) At low temperature and low press

i.ytimg.com/vi/otZvnZRQ65Y/sddefault.jpg

2.5 The Ideal Gas (Thermal Physics) (Schroeder)

Physics 32 Kinetic Theory of a Gas (10 of 10) Time Between Collision

Kinetic Theory of an Ideal Gas: Equation, Assumption, Concept


LBM Lecture 19: Chapman-Enskog expansion (part 1)

01 Kinetic Theory of Gases Theory1

Boyle's Law - A Level Physics
vt.physics

state the assumptions of kinetic theory of gases