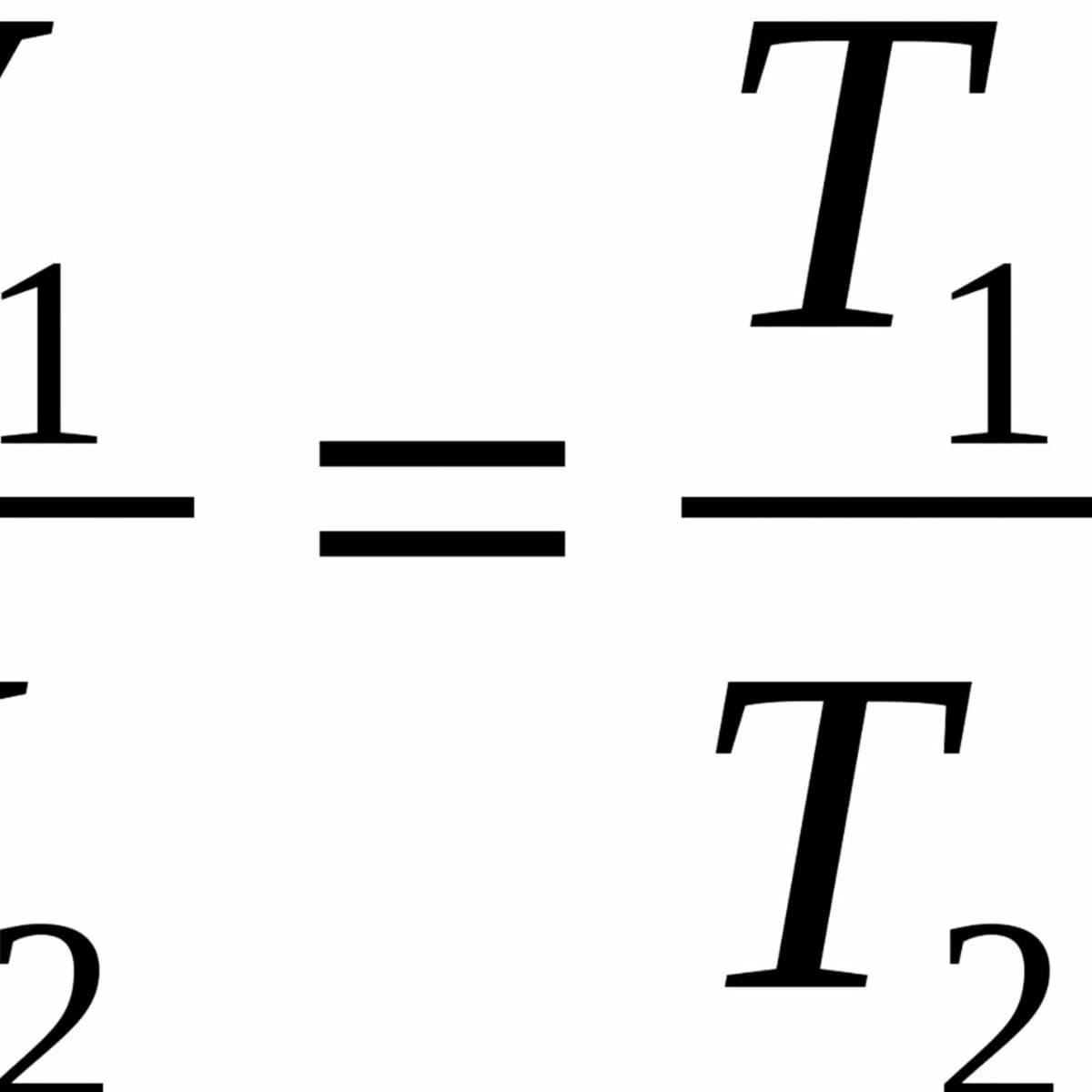Ideal Gas Law: Doubling Temperature and Volume

If pressure and temperature of an ideal gas are doubled and volume is halved, the number of molecules of gas.Become halfBecome four timesBecome two timesRemain constant
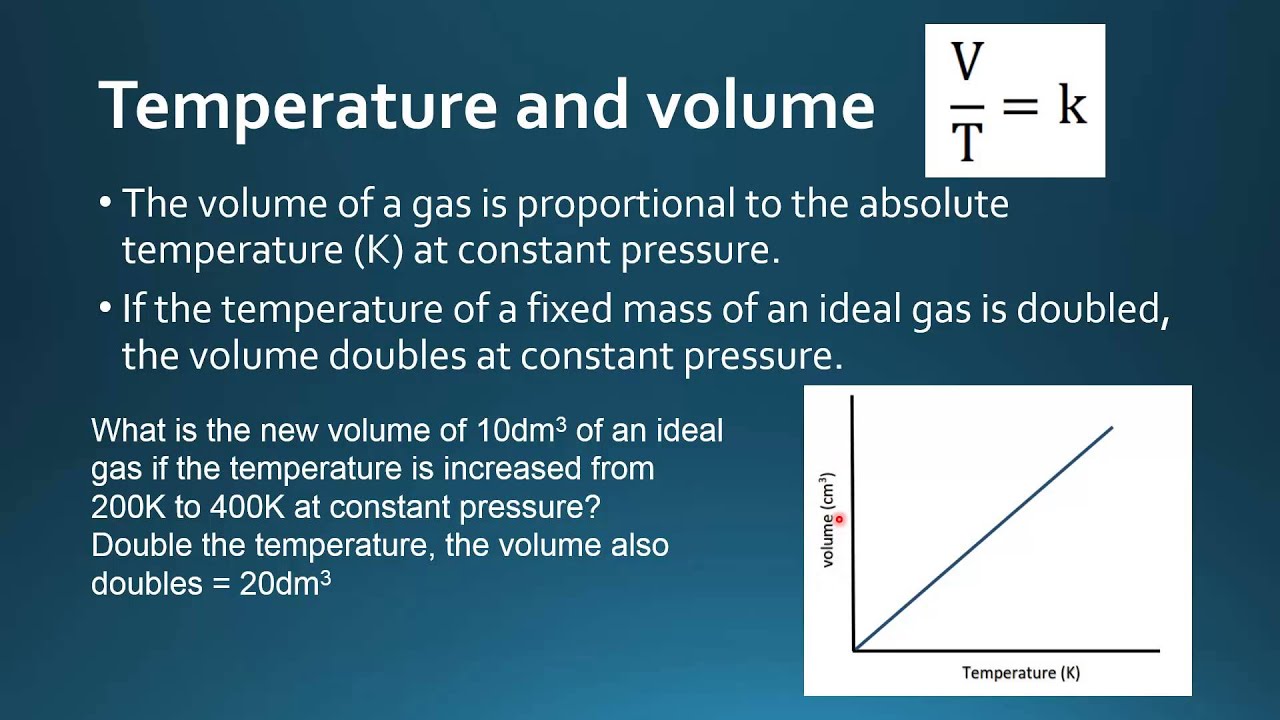
1.4.6 Solve problems involving temperature, pressure and volume for an ideal gas.

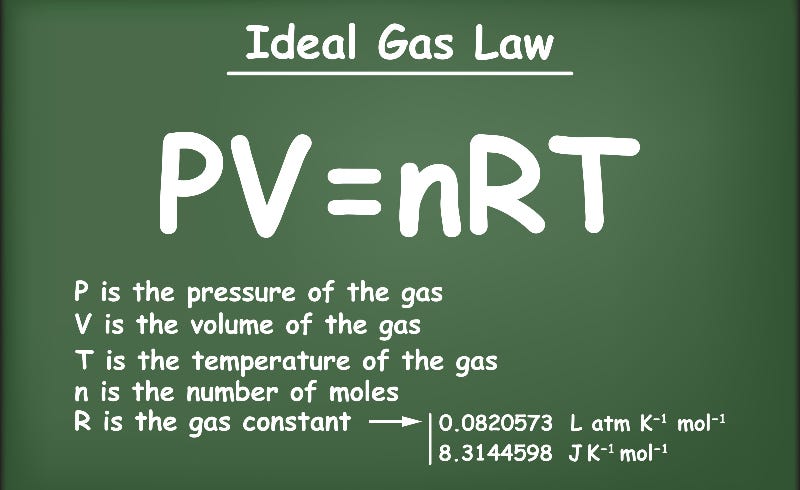
Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
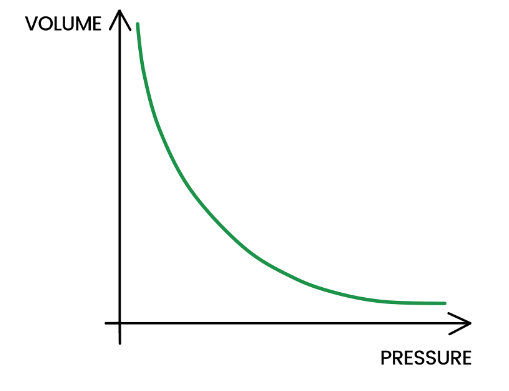
Gas Laws - Study Mind
Chapter 10 Gas Laws
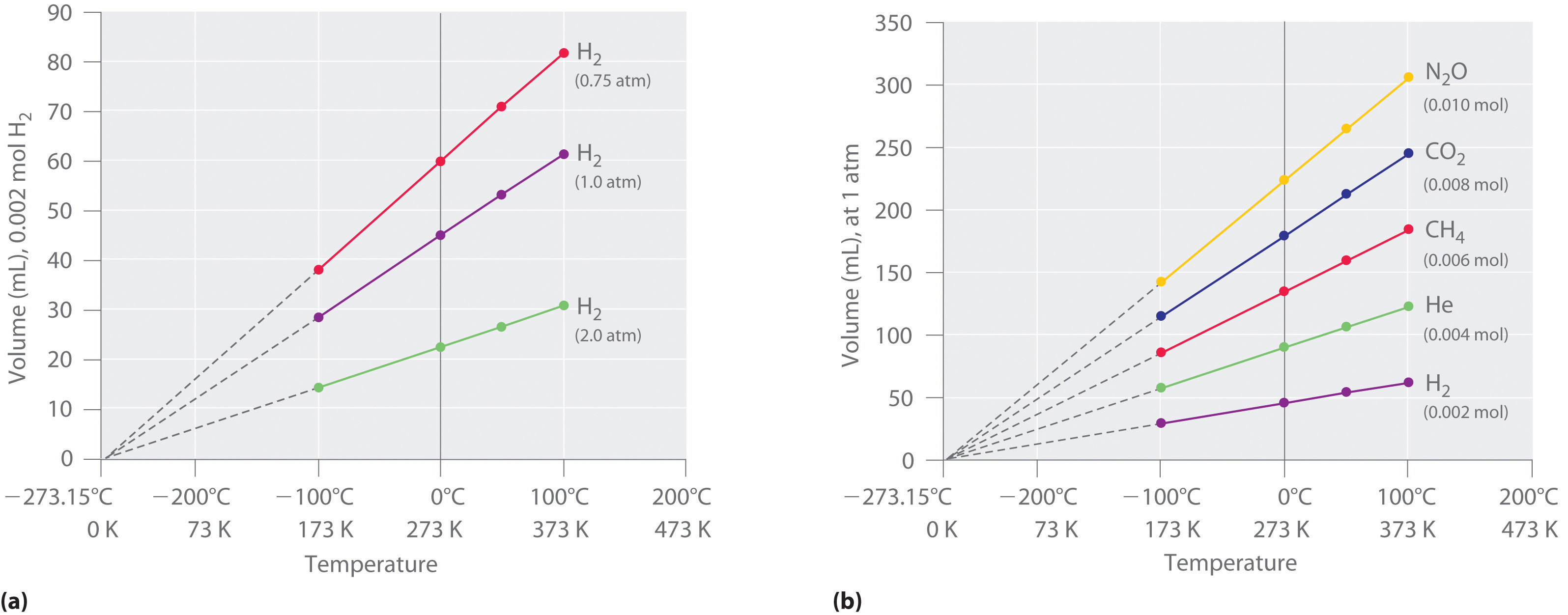
5.3: The Simple Gas Laws- Boyle's Law, Charles's Law and Avogadro's Law - Chemistry LibreTexts
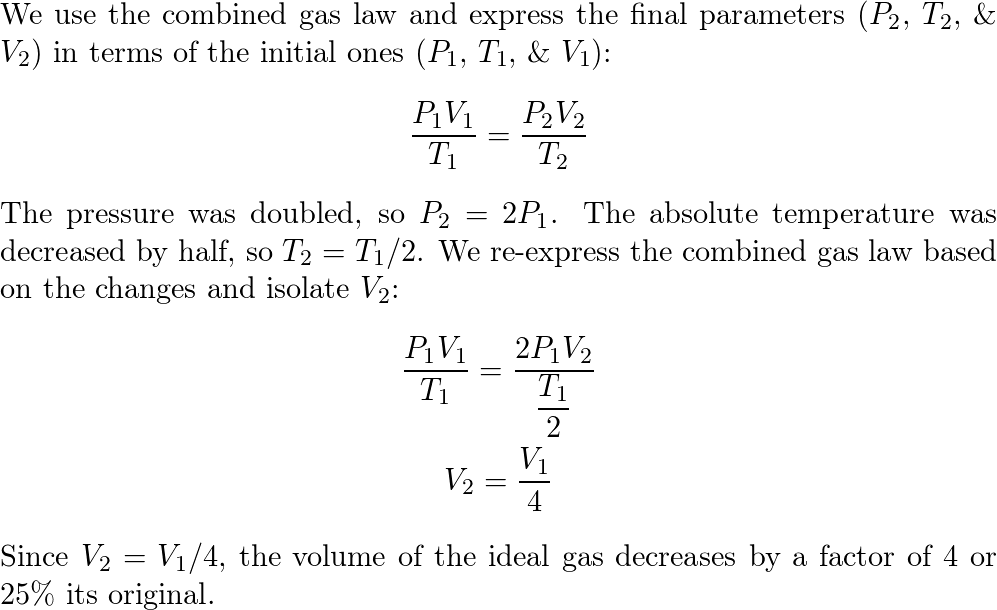
What will be the effect on the volume of an ideal gas if the

PDF) EXPERIMENT 5 IDEAL GAS LAW : CHARLES'S LAW
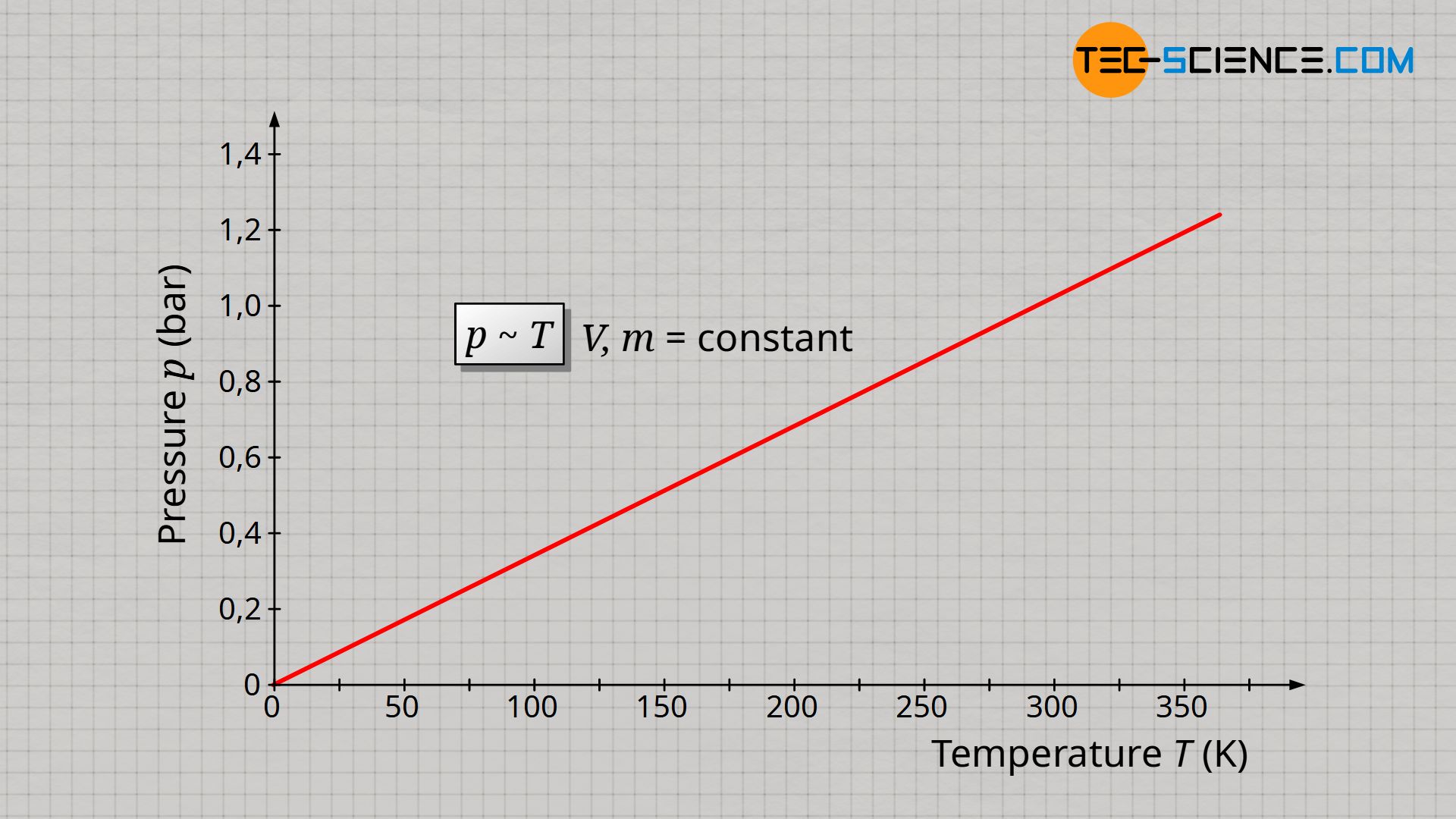
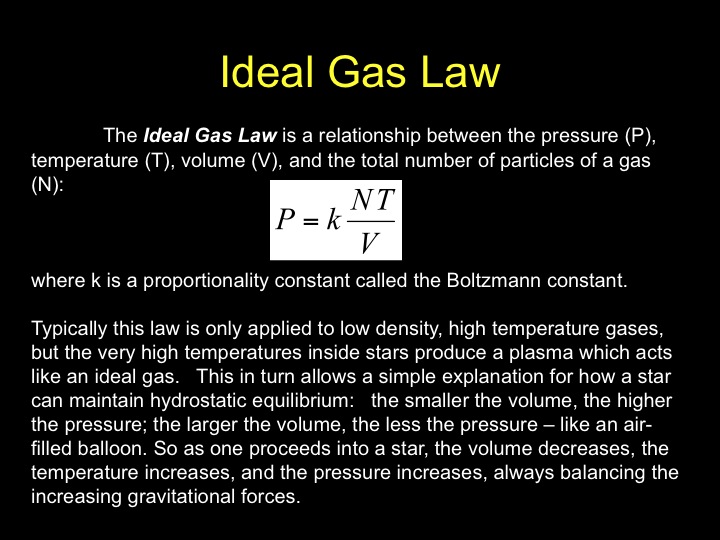
Ideal gas law (explained and derived) - tec-science
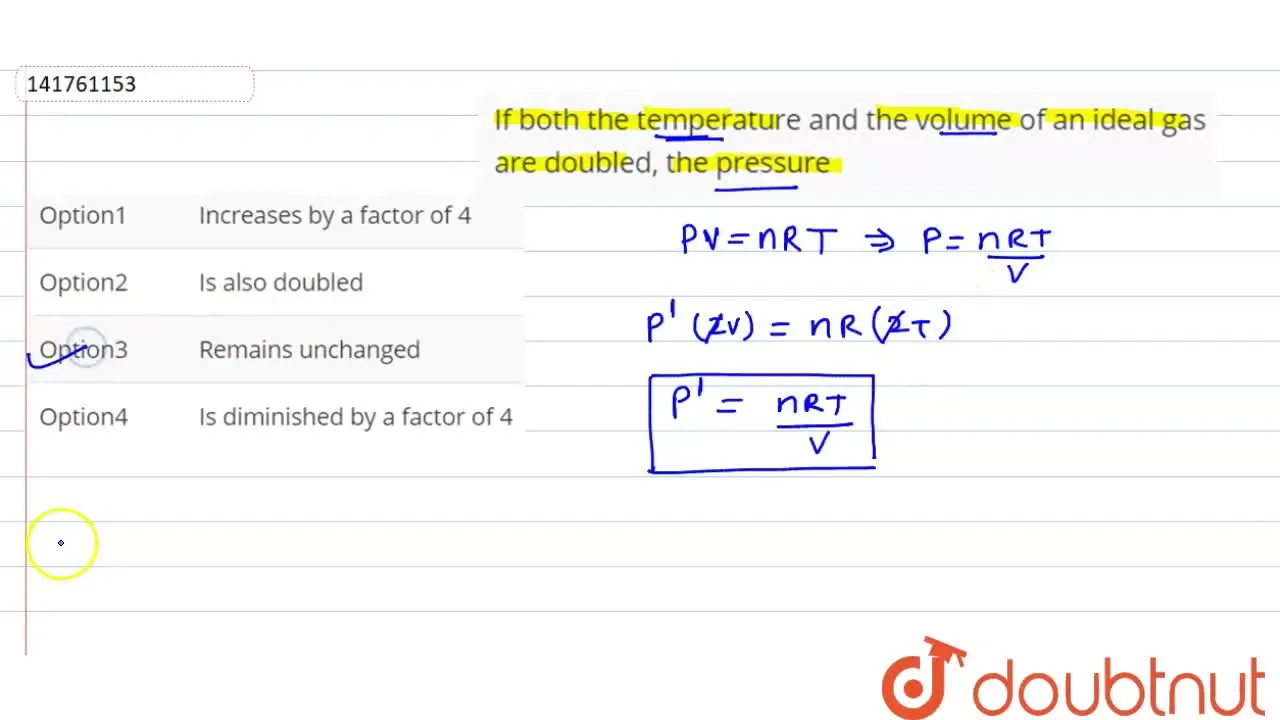
If both the temperature and the volume of an ideal gas are doubled, th
The ideal gas law (PV = nRT) (video)

Joule expansion - Wikipedia
Boyle
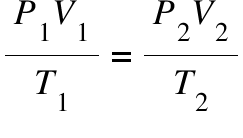
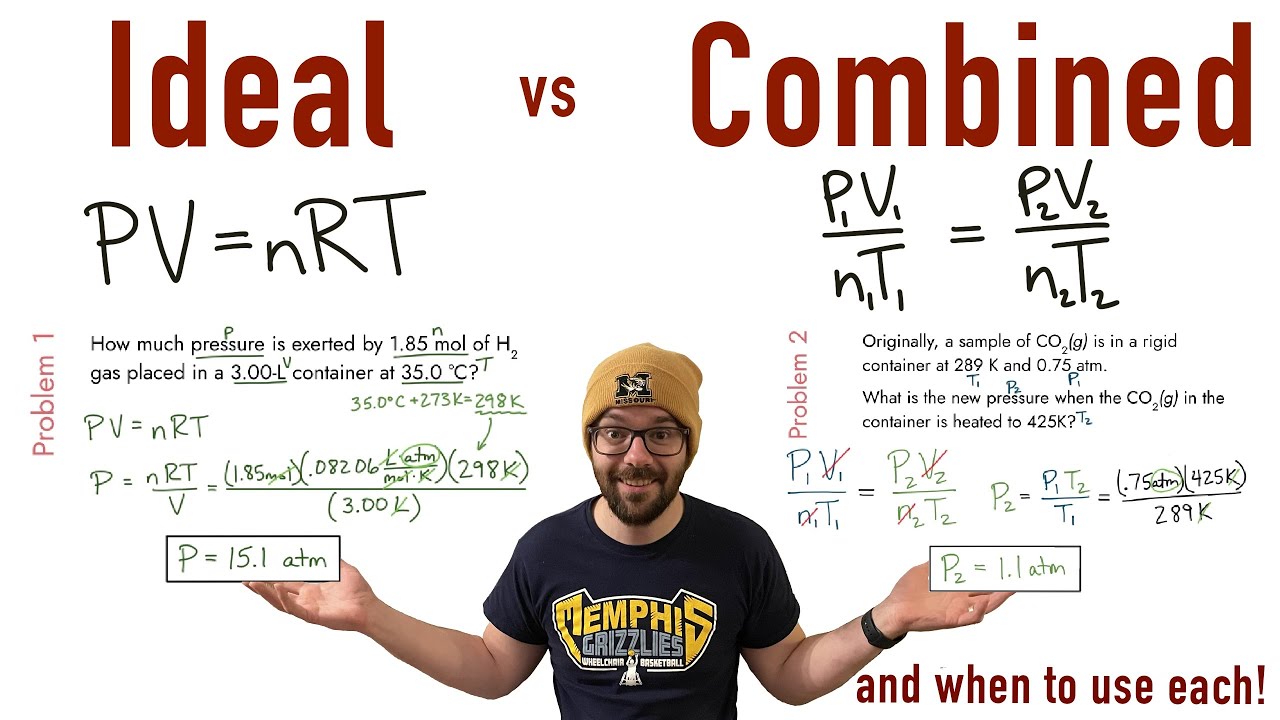
Combined Gas Law Calculator
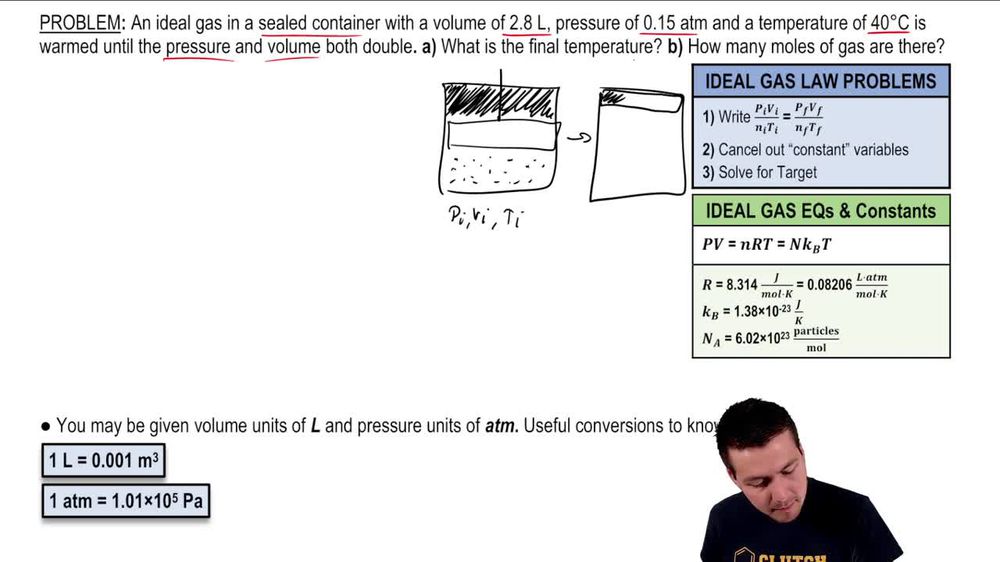
The Ideal Gas Law - Video Tutorials & Practice Problems