Modular Medical submits next-gen insulin pump for FDA clearance
Modular Medical (Nasdaq:MODD) announced today that it submitted its next-generation MODD1 insulin pump to the FDA for 510(k) clearance.

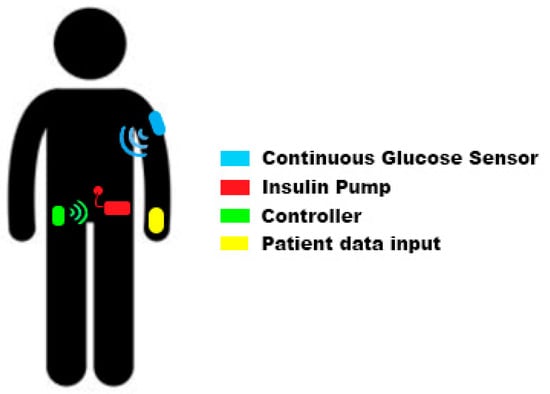
A Century of Diabetes Technology: Signals, Models, and Artificial Pancreas Control: Trends in Endocrinology & Metabolism

ex99_1page004.jpg
Electronics, Free Full-Text

Novo Nordisk inks diabetes dev partnership with Lyfebulb - MassDevice

These diabetes devices are set to launch in 2024

Erica Scott - CSAM on LinkedIn: Modular Medical submits next-gen insulin pump for FDA clearance

PAD: Medtronic launches HawkOne directional atherectomy device - MassDevice

Acutus Medical wins CE Mark for AcQMap image & mapping system - MassDevice

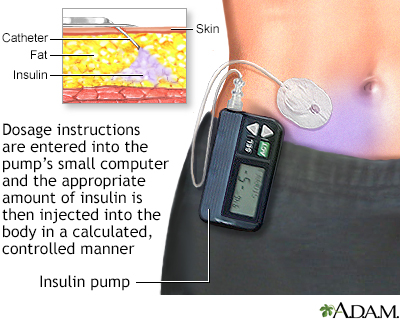
Automated insulin delivery: benefits, challenges, and recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association

David Kliff on LinkedIn: Modular Medical submits next-gen insulin pump for FDA clearance

David Kliff on LinkedIn: Beta Bionics earns pharmacy benefit win for bionic pancreas
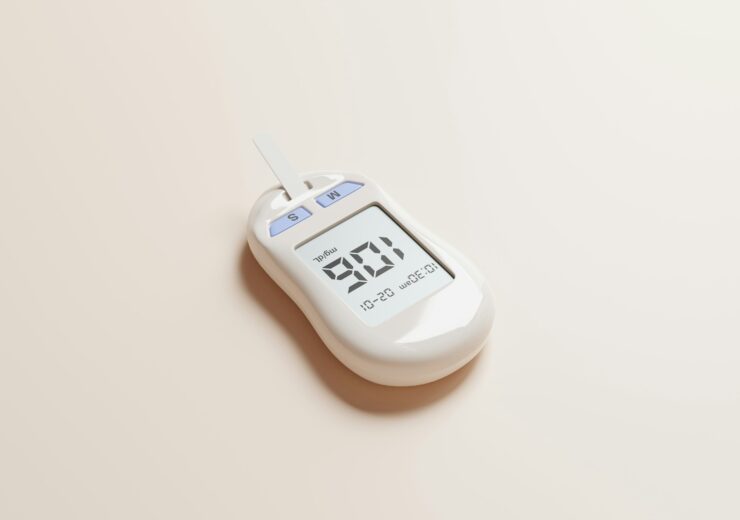
Modular Medical seeks FDA 510(k) clearance for MODD1 insulin pump

as-ex99_1page001.jpg