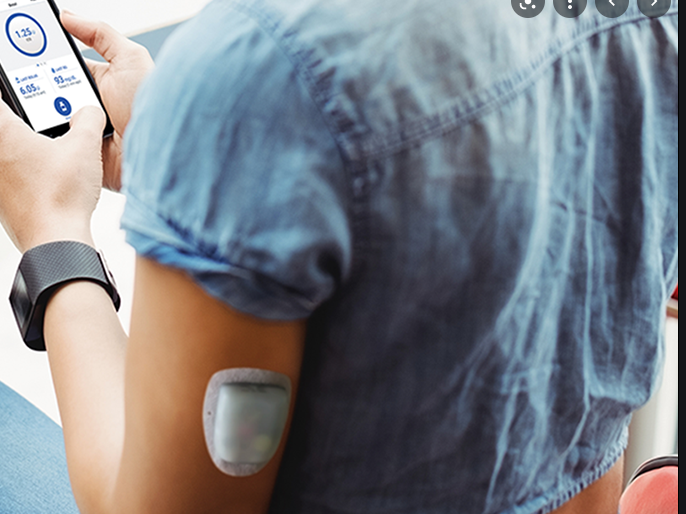
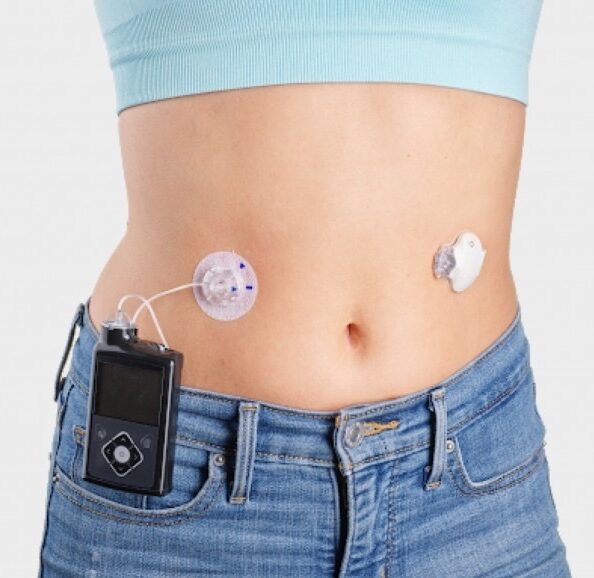
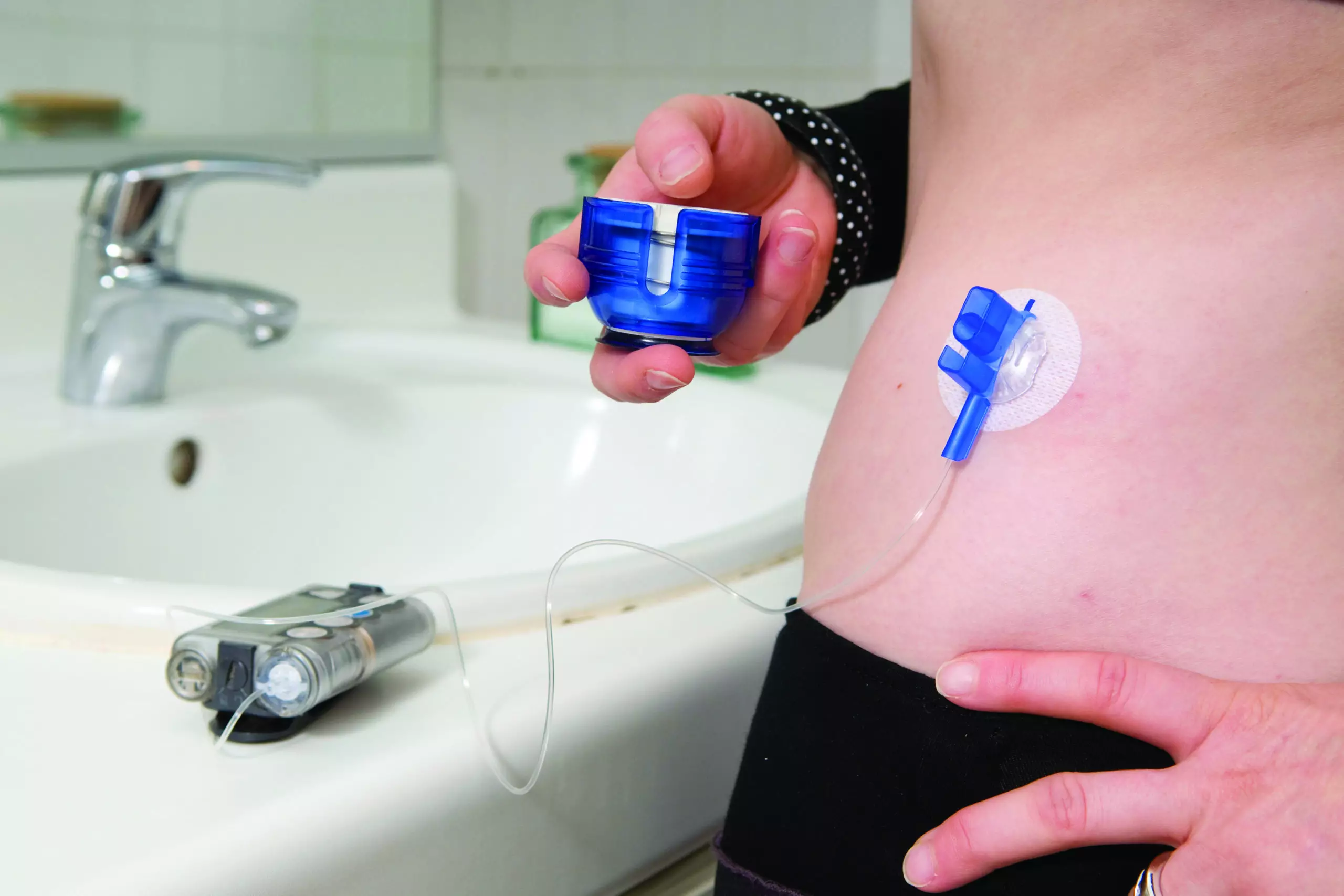
FDA says Medtronic MiniMed insulin pump recall is serious - MassDevice
The U.S. FDA has designated a recall of hundreds of thousands of Medtronic Minimed insulin pumps as Class I — the most serious type of recall. Medtronic (NYSE:MDT) first warned of safety problems with the pumps in November. The recall involves 322,005 pumps — MiniMed 630G (model MMT-1715) and MiniMed 670G (model MMT-1780) — in the […]

Medtronic Diabetes Pump Lawsuit - MiniMed Insulin Pump

Food & Drug Administration (FDA) Archives - Page 5 of 84 - Drug Delivery Business

Investors sue Medtronic for failing to disclose insulin pump

B. Braun infusion pump battery recall is Class I

MiniMed 780G System – P160017/S091

Baxter recalls some syringe pumps due to underdosing risk

Medtronic Diabetes Pump Lawsuit - MiniMed Insulin Pump

Eitan Medical's Sapphire infusion pump recall is Class I

Medtronic insulin pump recall: FDA says hackers could hijack