Microbial Culture Media For Quality Control Of Non-Sterile Products
lt;p>Using the correct media is critical to ensure microbiological quality. Explore a portfolio of culture media and substances for sample preparation, microbial enumeration tests, and tests for specified microorganisms.</p>

Culture Media for Compendial Methods
Microbial Culture Media- Definition, Types, Examples, Uses
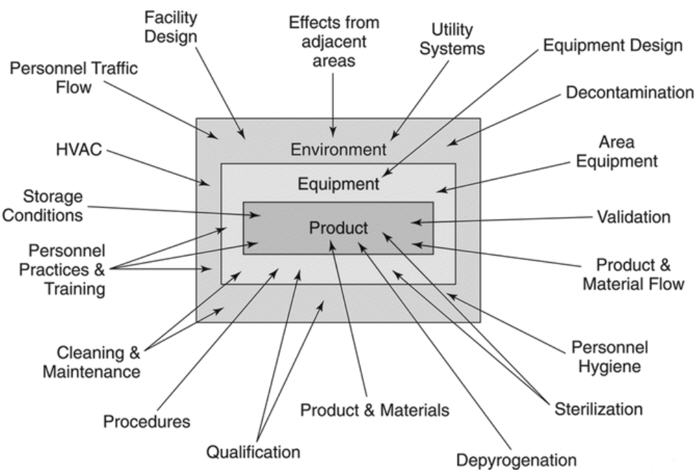
The Essential Components Of A Sterility Assurance Program

The Steritest System — Benchmark Technology For Filtration-Based Sterility Testing
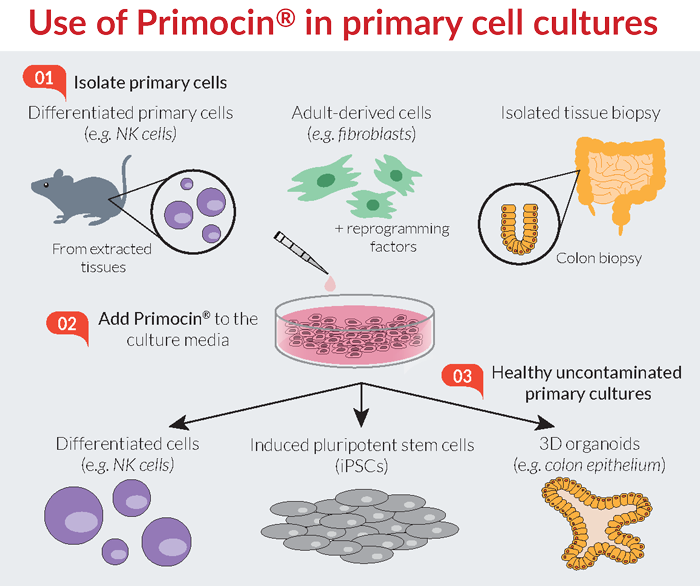
Primocin, Antimicrobial Reagent for Primary Cells

Microbial Culture Media

Facts about Environmental Isolates and Growth Promotion Test American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology

Bacteriology Culture Guide
Aseptic Technique Thermo Fisher Scientific - CA
High Complexity Media-Fill Test Kit - IVQA

Quality Control for Microbiological Culture Media - ppt video online download

The Steritest System — Benchmark Technology For Filtration-Based Sterility Testing