
GE HealthCare Receives FDA Clearance for Portrait Mobile, A First-Of-Its-Kind, Wireless Monitoring Solution Aiding Early Detection of Patient Deterioration
4.6
(701)
Write Review
More
$ 16.00
In stock
Description

Portrait™ Mobile GE HealthCare (United States)

PDF) Towards wearable sensing-based precise and rapid responding system for the early detection of future pandemic

Brad Townsend on LinkedIn: #proudtobeabbott

MedTech News Viseon, ZimVie, Acorai, CytoSorbents, GE, 4WEB

Viome Closes $86M BSCA Drops CVS - Digital Health Wire
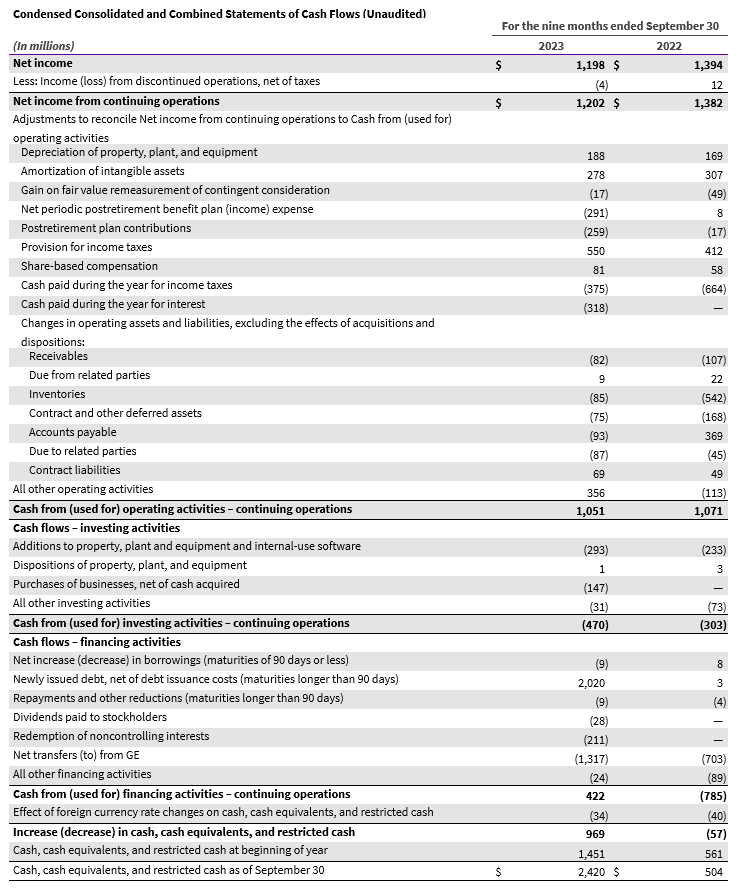
GE HealthCare Reports Third Quarter 2023 Financial Results

Greg Kalavas posted on LinkedIn

Brad Townsend on LinkedIn: #abbott

GE HealthCare Portrait Mobile patient monitoring device receives FDA clearance

Jeff Vekony on LinkedIn: We're very happy to share this news that

GE HealthCare scores 510(k) clearance for maternal and fetal monitoring platform
Related products
You may also like








