NGAL - Bioporto
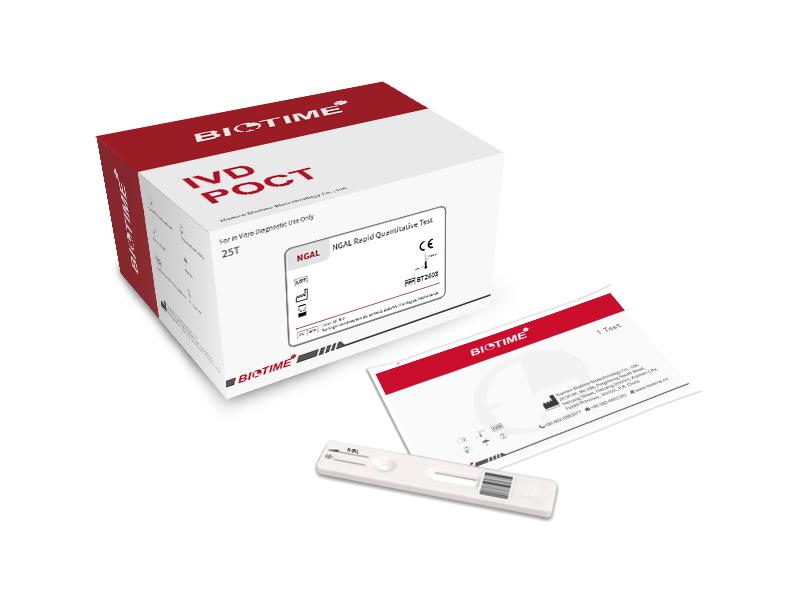
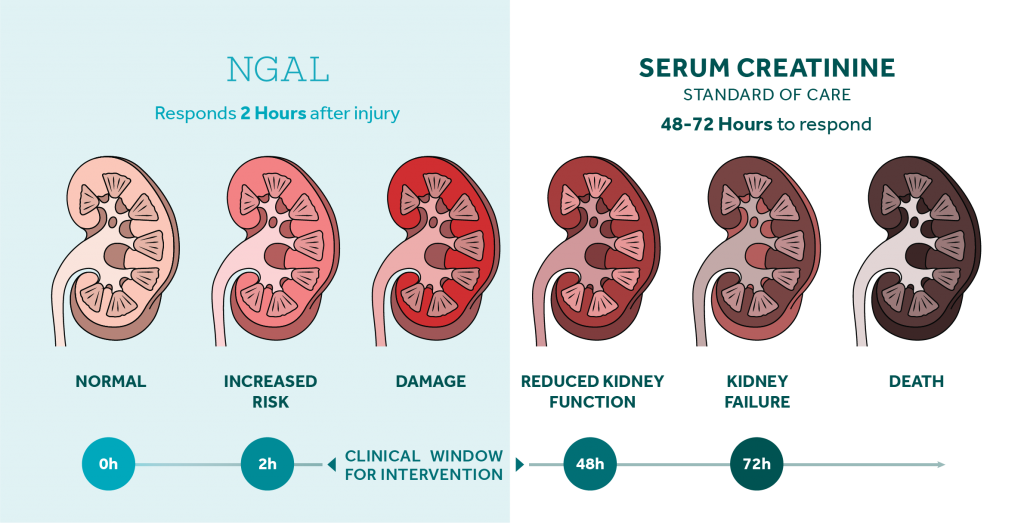
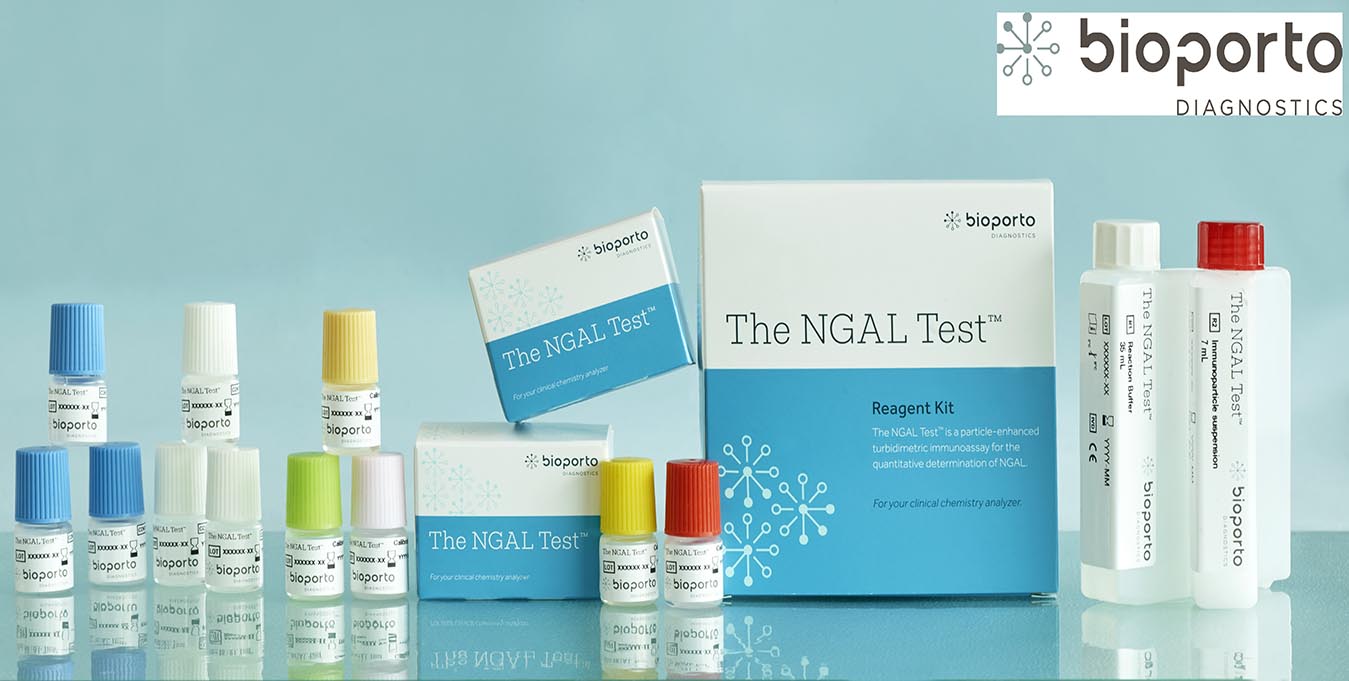
The NGAL Test is a particle-enhanced turbidimetric immunoassay for the quantitative determination of NGAL in human urine and plasma on automated clinical chemistry analyzers. NGAL measurements are useful in the risk assesment of AKI.

PDF) Cost-effectiveness and value of information analysis of NephroCheck and NGAL tests compared to standard care for the diagnosis of acute kidney injury
Clonezyme Biotek – Aflatoxin Kits, Antibiotic Elisa Kits, NGAL Test Kits, Bacterial Antisera, QBC Malaria System, USFDA EPA approved Disinfectant, Interscience Bag Mixer, Colony counters, Best, Top, Dealer, Supplier, Wholesaler, Importer, Distributor
Clonezyme Biotek

BioPorto Diagnostics A/S

Elevated Neutrophil Gelatinase-Associated Lipocalin Is Associated With the Severity of Kidney Injury and Poor Prognosis of Patients With COVID-19 - ScienceDirect

Kidney Tubular Damage and Functional Biomarkers in Acute Kidney Injury Following Cardiac Surgery - ScienceDirect

Identification of Urinary Activin A as a Novel Biomarker Reflecting the Severity of Acute Kidney Injury
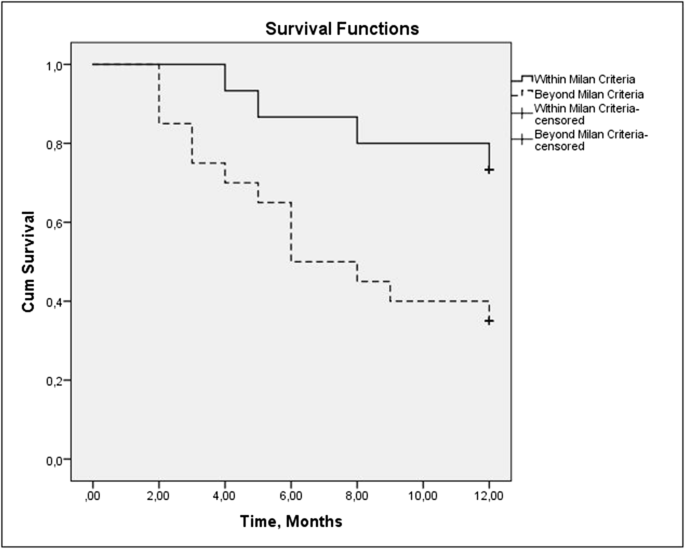
May Neutrophil Gelatinase-Associated Lipocalin (NGAL) Level Predict Mortality in Patients with Hepatocellular Carcinoma (HCC)?

Elevated Neutrophil Gelatinase-Associated Lipocalin Is Associated With the Severity of Kidney Injury and Poor Prognosis of Patients With COVID-19 - ScienceDirect

Home - Bioporto US

News - Bioporto