ClearPoint Neuro Announces FDA Clearance and First-in-Human Cases
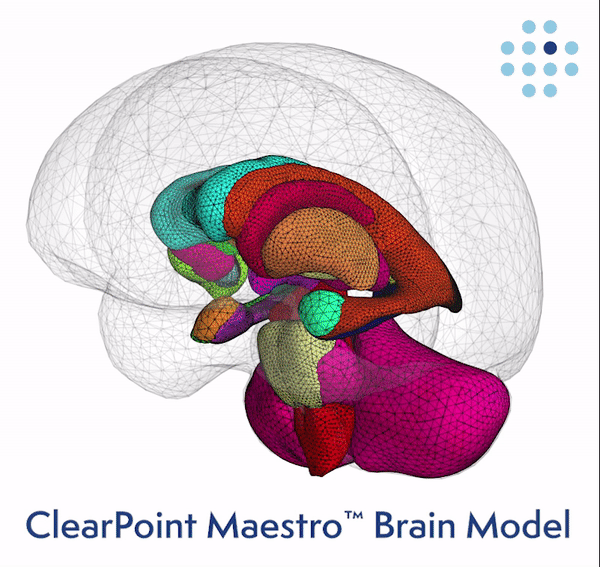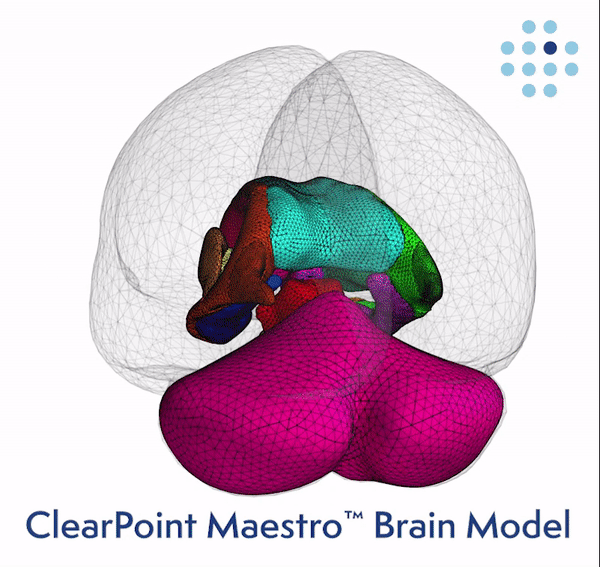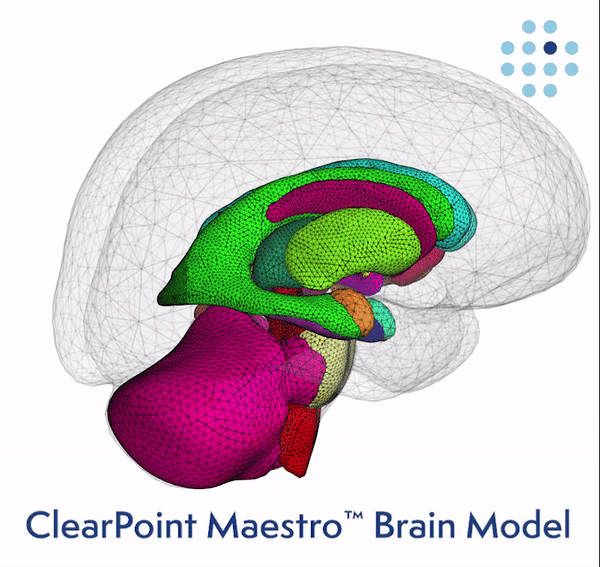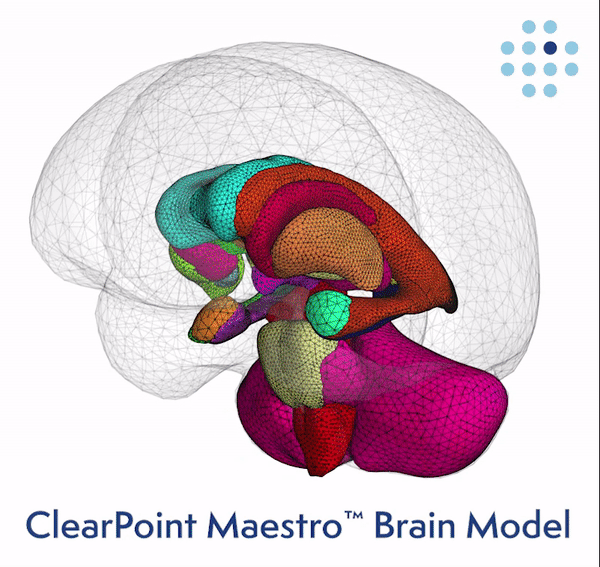
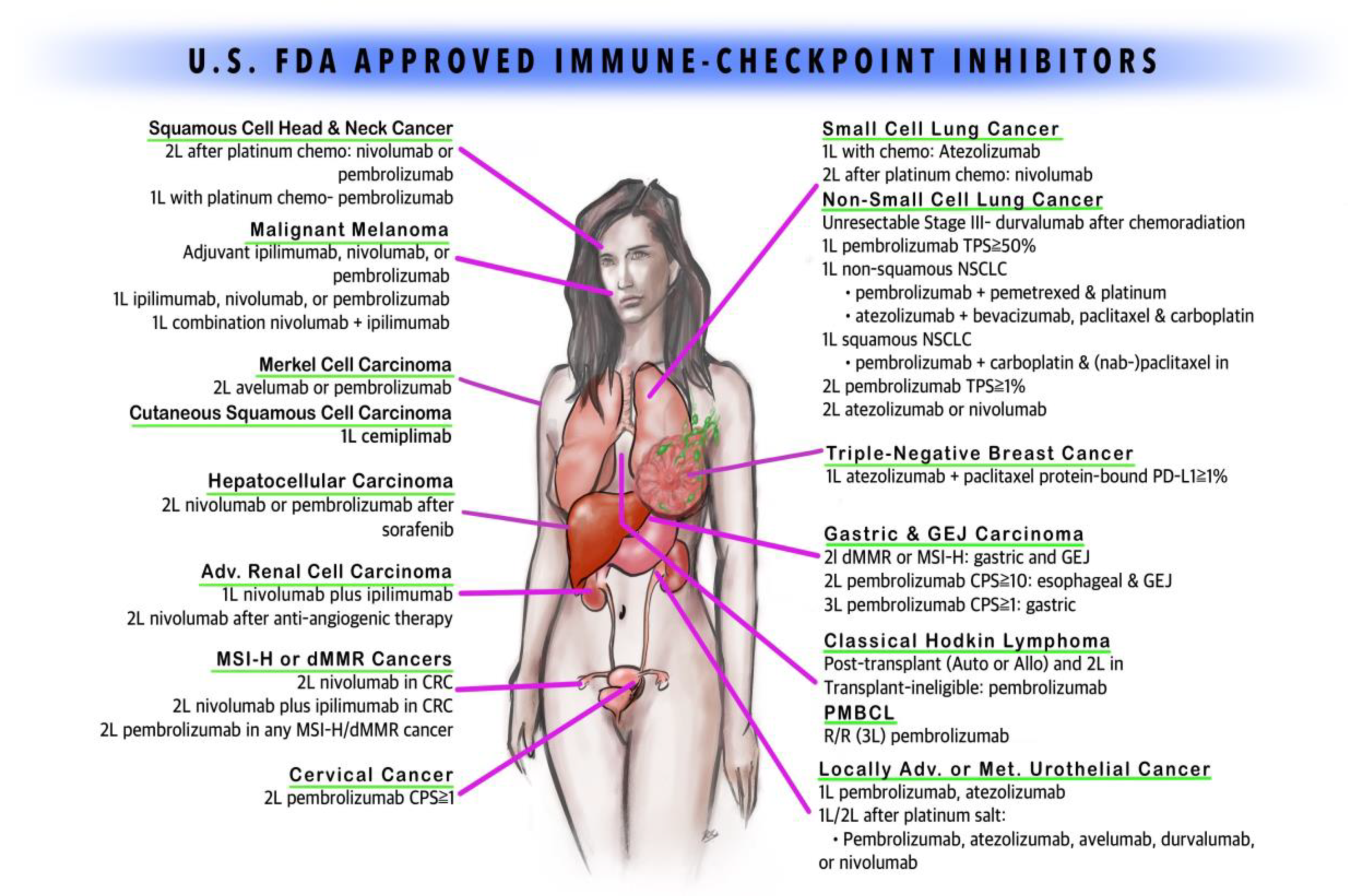
Clinical Validation of the Brain Model Published Online in the Journal NeuroImage ClearPoint Maestro Brain Model ClearPoint Maestro Brain Model ClearPoint Neuro Maestro Brain Model Comparative Reproducibility Error Results Reproducibility error of Maestro (green), FreeSurfer 7.2 (blue) and manual segmentation (red) for common brain structures (left), and average over all structures (right). Bars represent range of measured relative volume difference. SOLANA BEACH, Calif., Feb. 21, 2024 (GLOBE NE
Clinical Validation of the Brain Model Published Online in the Journal NeuroImage ClearPoint Maestro Brain Model ClearPoint Maestro Brain Model ClearPoint

ClearPoint Neuro, Inc. Announces First Procedure Utilizing ClearPoint® 2.0 Software in Europe
ClearPoint Neuro, Inc. Announces FDA Clearance and First-In-Human Cases Performed with the New 2.2 Software Version and the Integrated Maestro Brain Model -February 21, 2024 at 04:05 pm EST

ClearPoint Neuro wins FDA nod for ClearPoint 2.2 with Maestro Brain Modeling
Cancers, Free Full-Text
ClearPoint Neuro Announces FDA Clearance and First-in-Human
ClearPoint Neuro Announces FDA Clearance for ClearPoint Prism™ Neuro Laser Therapy System :: ClearPoint Neuro, Inc. (CLPT)

HealthTech Industry News and Analysis

RapidAI snags FDA clearance for neuroimaging analysis device

SNN
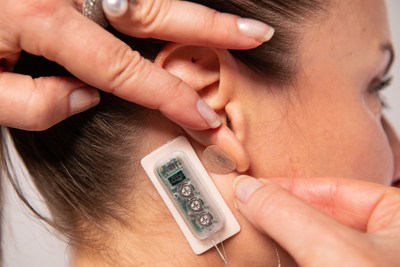
FDA Clears DyAnsys Neurostimulation Device Primary Relief to Treat Post-Cardiac Surgery Pain

Stock Market News 2024-02-21









