
Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt

Compressibility factor - Wikipedia

3.3: Real gas and compressibility factor - Engineering LibreTexts

Gas compressibility factor Z: Ideal gas vs Real gas

Ideal Gas Law - an overview

Real gasses For an ideal gas, the compressibility factor Z = PV/nRT is equal to unity for all conditions. For a real gas, Z can be expressed as a function. - ppt
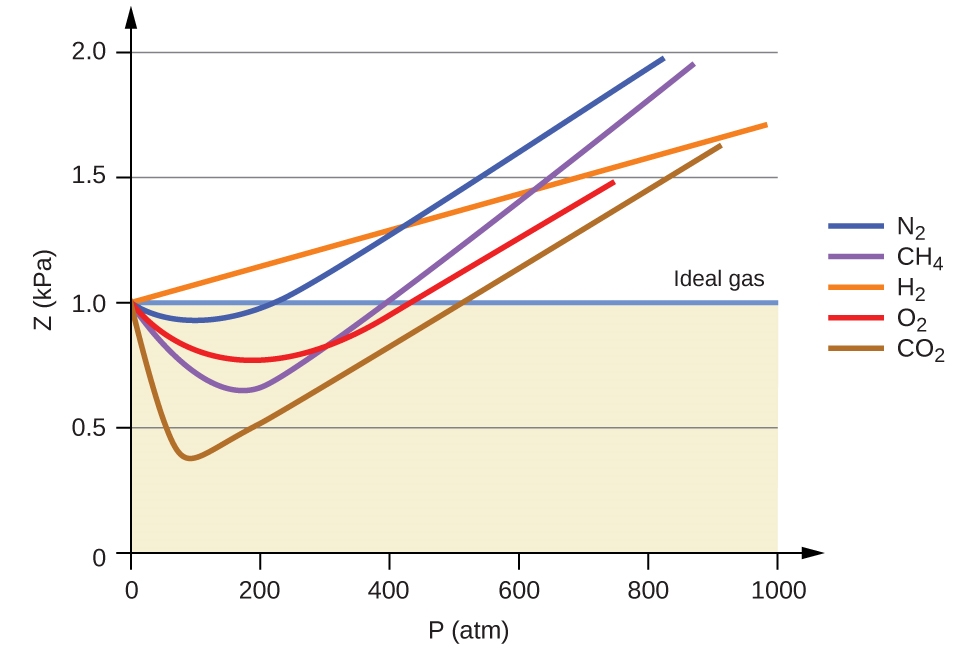
2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

Compressibility Factor of Gas Overview, Equation & Chart

Solved 4. Real gas effects can be expressed as departures

What is compressibility factor? What is its value for ideal gas

Slideshow chapter 1 3 physical chemistry 1 dr ngo thanh an

Real Gas Behavior The Compression Factor (Z) [Example #2]

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Properties of Gas Manik
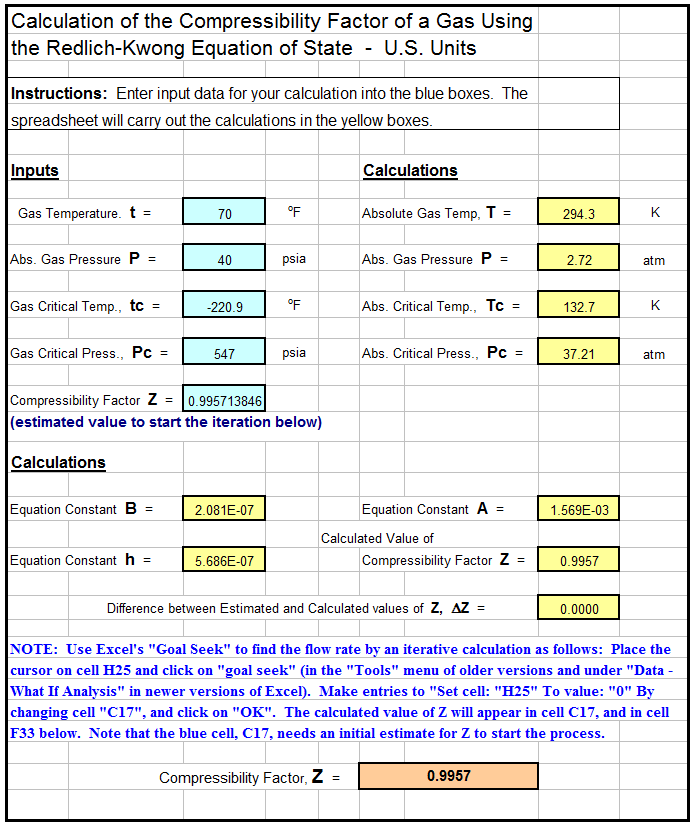







/product/32/0555922/1.jpg?8826)
