Quantum Numbers for Atoms - Chemistry LibreTexts
A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The combination of all quantum numbers of all electrons in an atom is …
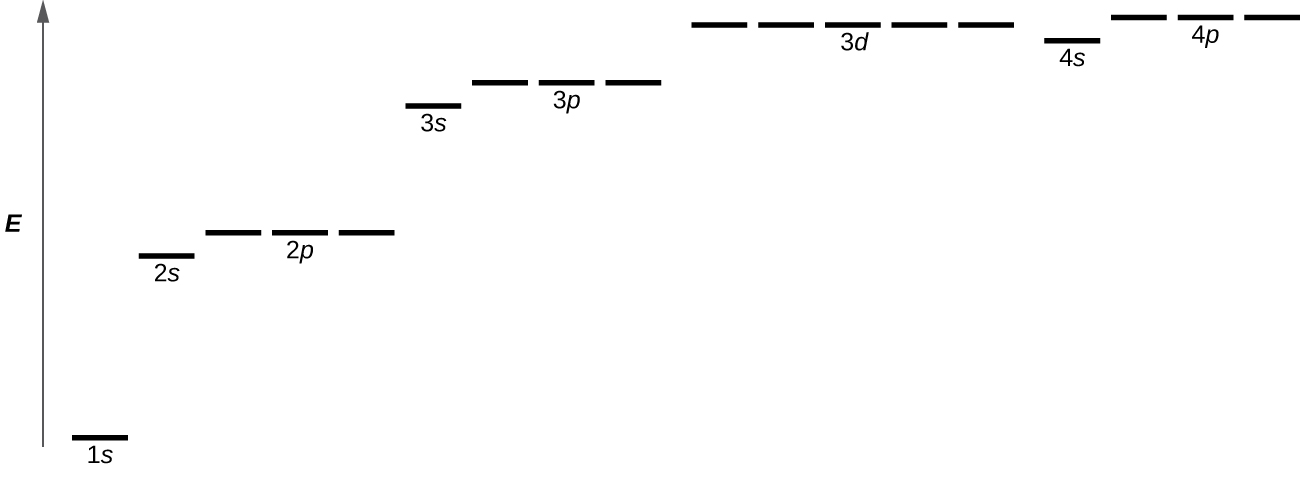
A total of four quantum numbers are used to describe completely the movement and trajectories of each electron within an atom. The combination of all quantum numbers of all electrons in an atom is described by a wave function that complies with the Schrödinger equation. Each electron in an atom has a unique set of quantum numbers; according to the Pauli Exclusion Principle, no two electrons can share the same combination of four quantum numbers.
3.3: Development of Quantum Theory - Chemistry LibreTexts

General Chemistry 1 Q2 Module-1, PDF
Family Of V(III)-Tristhiolato Complexes Relevant To, 46% OFF

Ionization energy - Wikipedia

9.A Condensed Matter Physics (Answers) - Physics LibreTexts

Impressions: Robinson's Brutus Awards For 2015, Part, 42% OFF
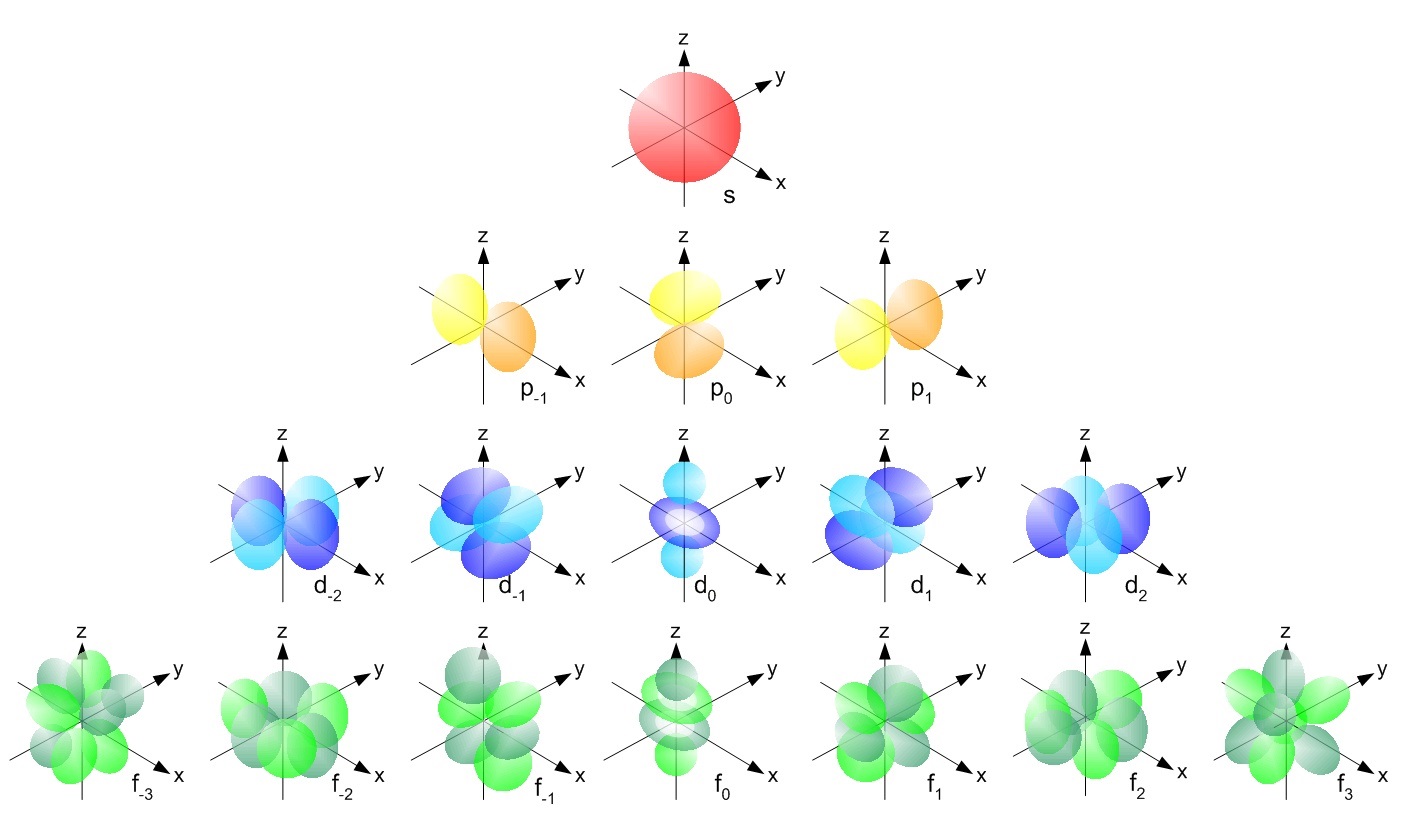
What represents the area in an atom where an electron most likely

GeneralChemistry1 Q2 Module-1 Quantum Mechanical Descriptions v5-1.pdf - Senior High School NOT General Chemistry 1 Quarter 2 - Module 1 Quantum

Pauli Exclusion Principle - Chemistry LibreTexts
This Little-Known Quantum Rule Makes Our Existence Possible

Subatomic particle, Definition, Examples, & Classes