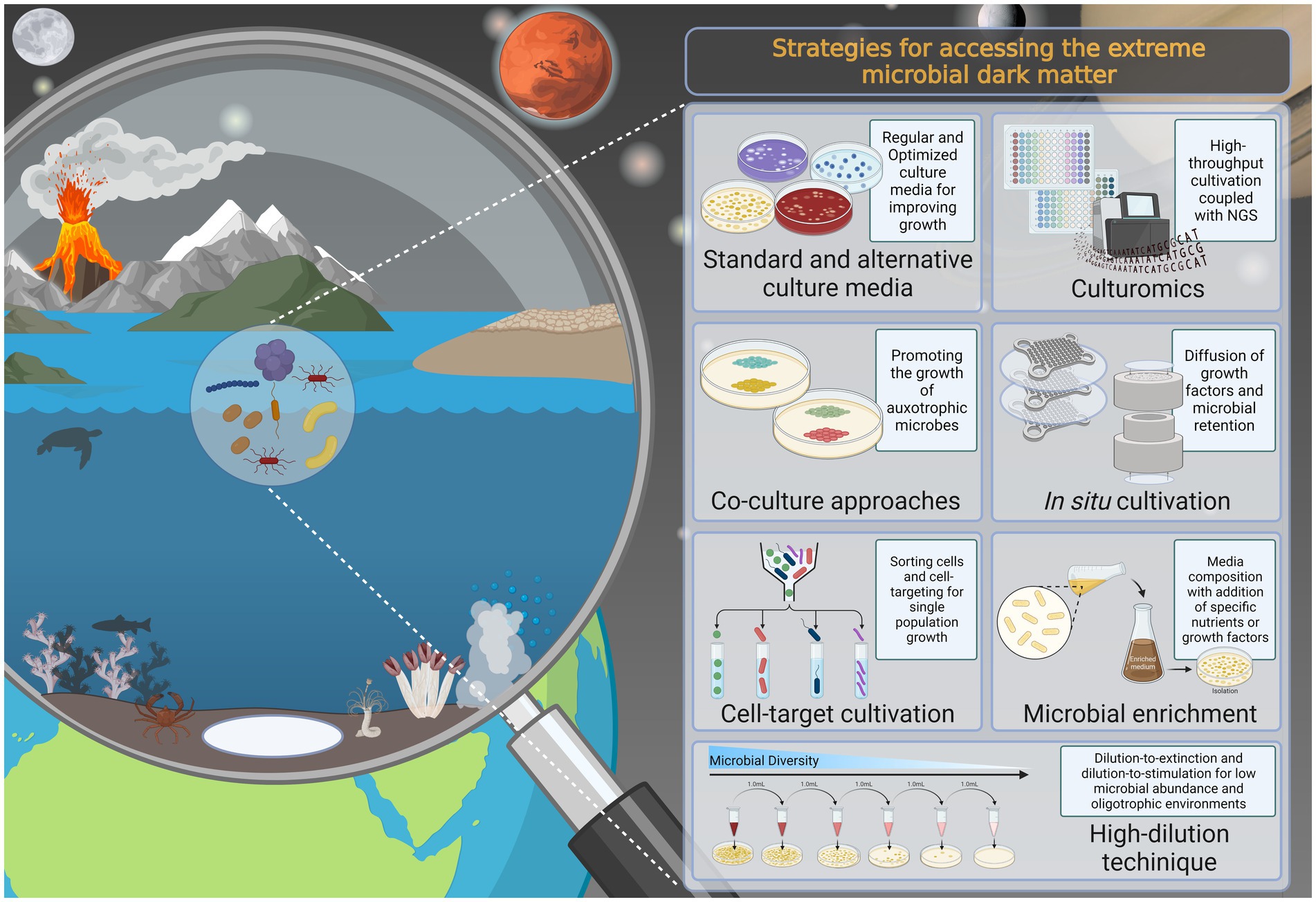
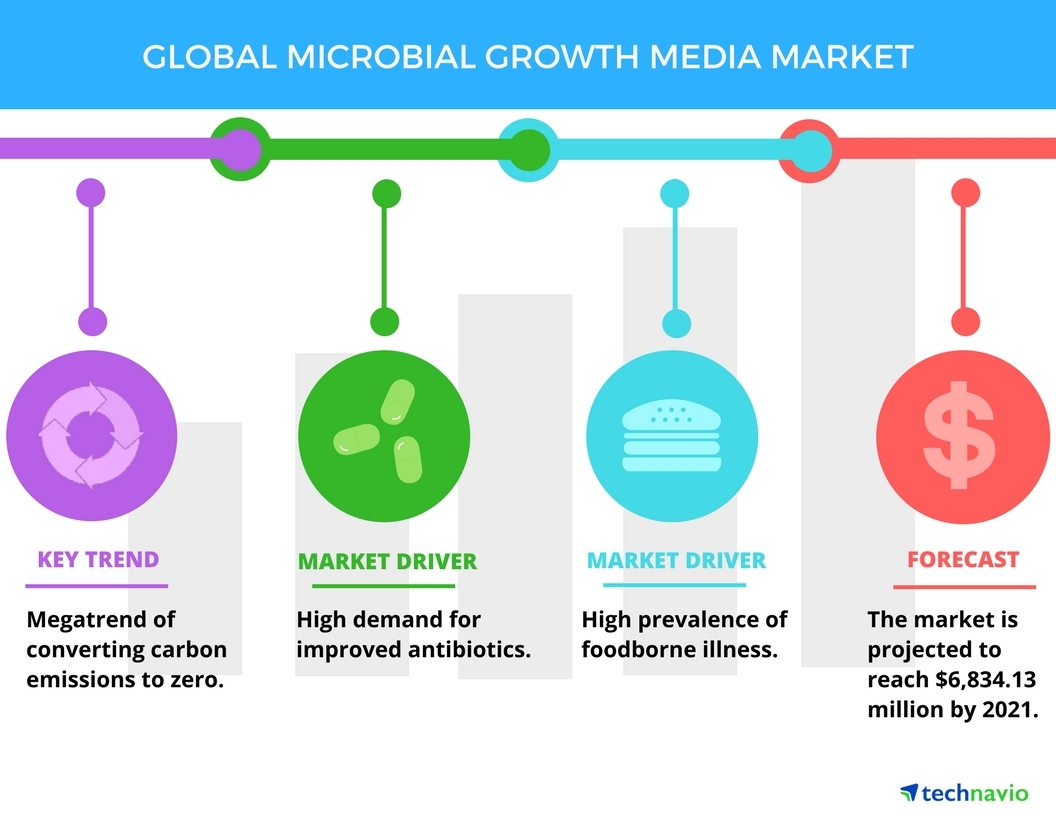
Microbiological Media Management - SOP & Guideline - Pharma Beginners
4.5
(395)
Write Review
More
$ 9.00
In stock
Description
Standard Operating Procedure (SOP) and Guideline for the Receipt, Storage, Preparation, Growth Promotion Test, use, and Disposal of microbiological media.
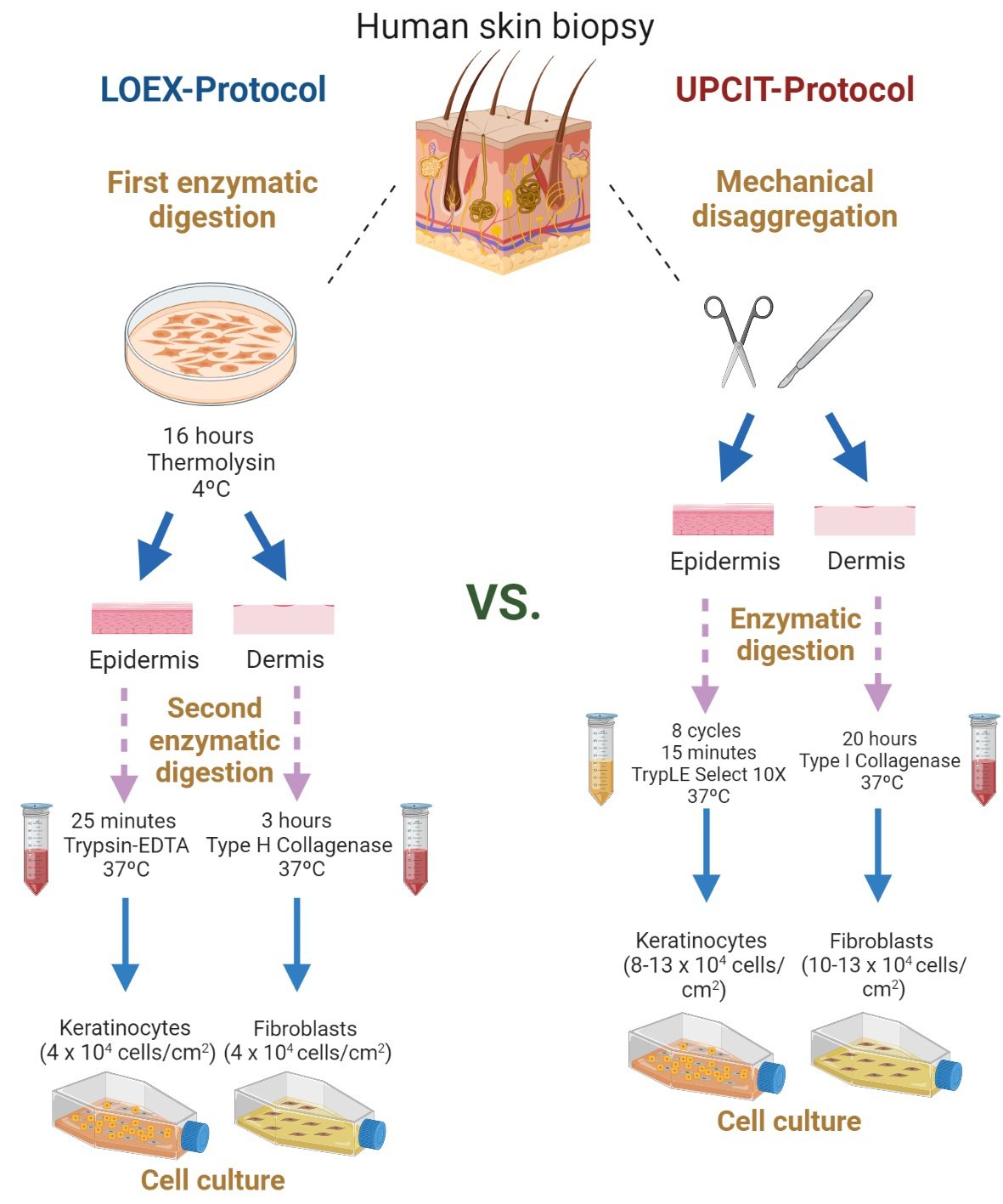
IJMS, Free Full-Text

The Importance of a Strong SOP System in the QC Microbiology Lab

EN ISO 11133 and Water for the Preparation and Performance Testing

Sterility Testing Units, Microbiology, Sterisart®

Culture Media Preparation
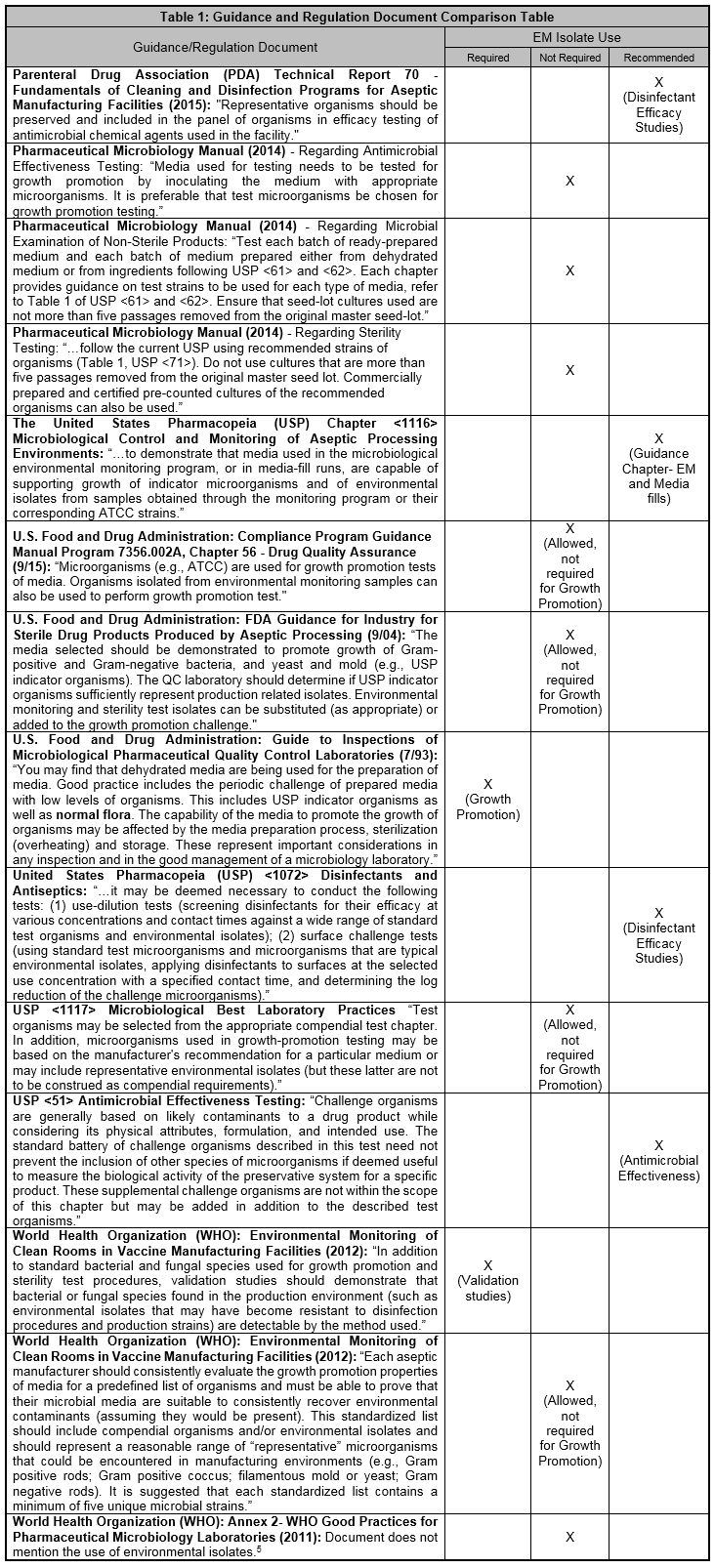
Environmental Isolates What's The Proper Use Of In-House Cultures
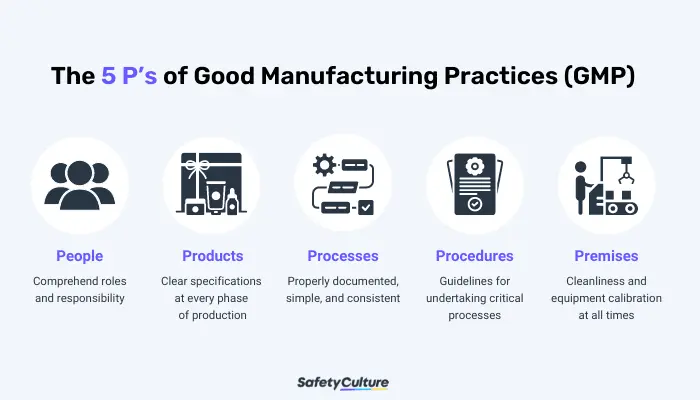
What is GMP, Good Manufacturing Practices

SOP For Microbiological Good Laboratory Practices
Diagnostics, Free Full-Text
Related products
You may also like