FDA Approves Senza®, Nevro's High Frequency Spinal Cord
The Senza System has been approved by the FDA for the treatment of chronic pain associated with painful diabetic neuropathy.

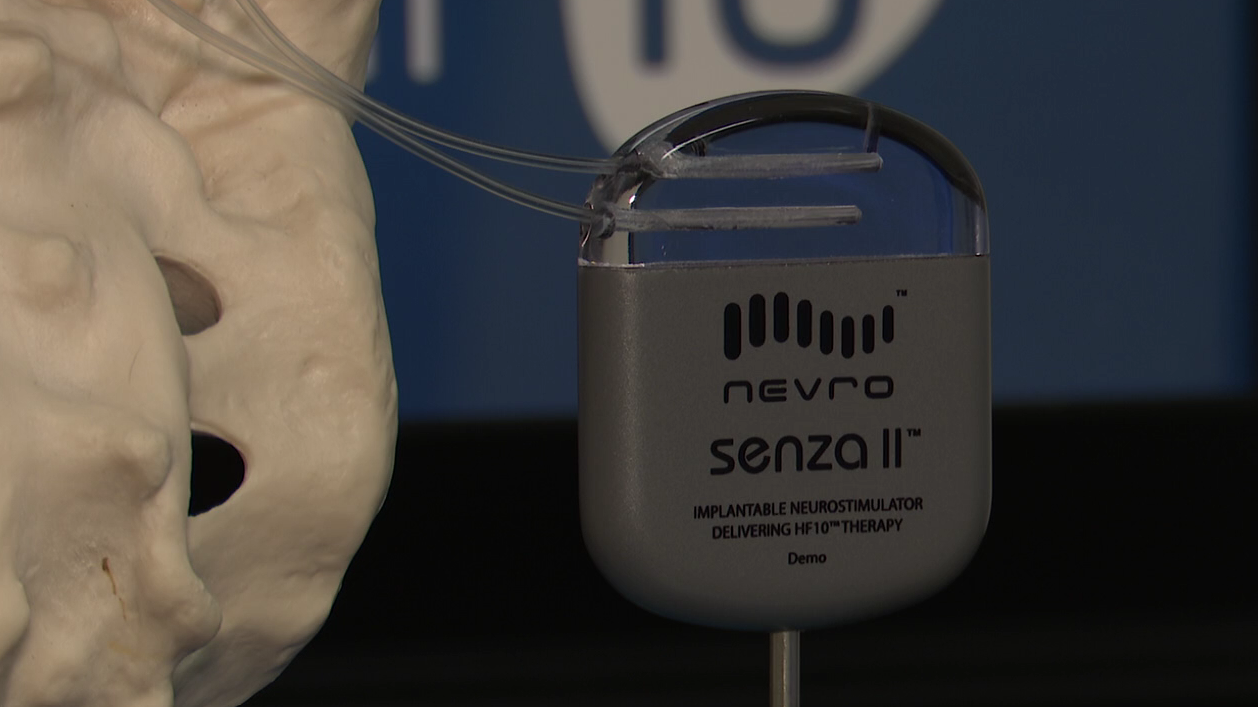
Nevro Receives FDA Approval For Senza II Spinal Cord Stimulation System Delivering HF10 Therapy

Carsen Messig on LinkedIn: FDA Approves Expanded Labeling for High-Frequency SCS for Nonsurgical…

Deck Review with Nevro - by Joshua Elkington - Axial

Nevro Corp. - AU - Providers - HFX for PDN

Nevro Earns CE Mark For Senza Omnia Spinal Cord Stimulation

Long-term efficacy of high-frequency (10 kHz) spinal cord stimulation for the treatment of painful diabetic neuropathy: 24-Month results of a randomized controlled trial - ScienceDirect
Senza Archives - Beyond Type 1

nvro-10k_20181231.htm
Despite successes, lack of regulation raises concerns over medical devices
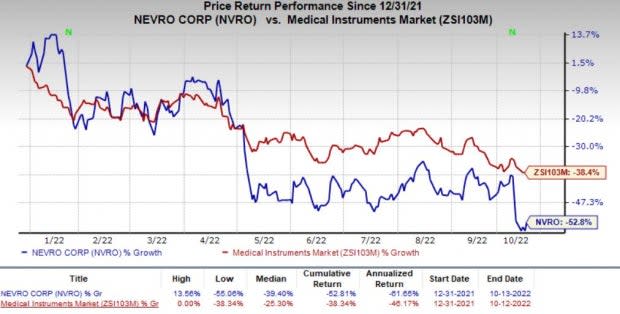
Nevro (NVRO) Gains Following FDA Approval for Senza HFX iQ
HF10 Therapy Shown Effective For Variety of Pain Conditions

Leadership Through Innovation Tm - ppt video online download








)
