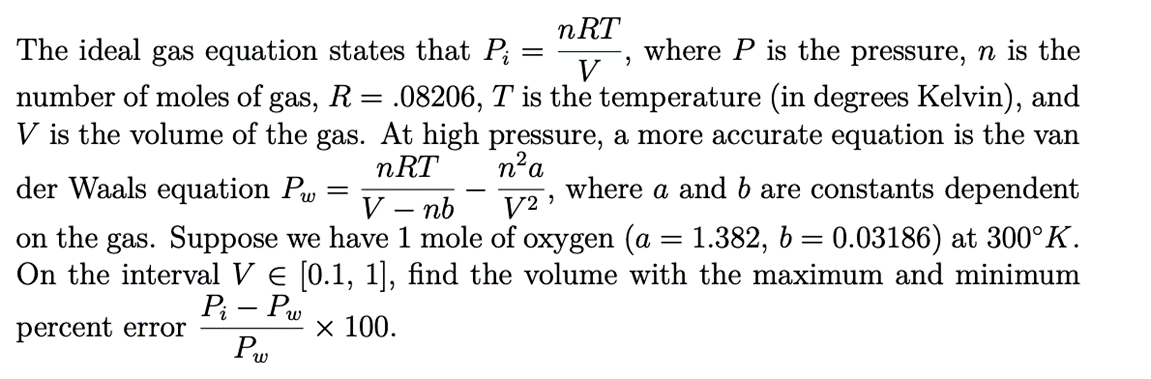
Solved) - NRT The Ideal Gas Equation States That Pi Where P Is The Pressure, (1 Answer)
amp;#160;NRT The Ideal Gas Equation States That Pi Where P Is The Pressure, N Is The V Number Of Moles Of Gas, R= .08206, T Is The Temperature (In Degrees Kelvin), And V Is The Volume Of The Gas. At High Pressure, A More Accurate Equation Is The Van NRT

SOLUTION: Ideal gas law worksheet 2 answer - Studypool

The graph below shows the change in pressure as the temperature i

Surface pressure versus area per molecule for an ideal gas model.
The ideal gas law (PV = nRT) (video)

Osmosis and Osmotic Pressure: Definition, Formulas, Proof, Videos, Q&A

OpenStax College Physics, Chapter 13, Problem 24 (Problems
How to use the ideal gas law to find volume - Quora
Solved PV RT The ideal gas equation states that = n, where P
Processes, Free Full-Text
How to calculate the values of critical pressure and temperature for a given gas (Van der Waals equation) - Quora