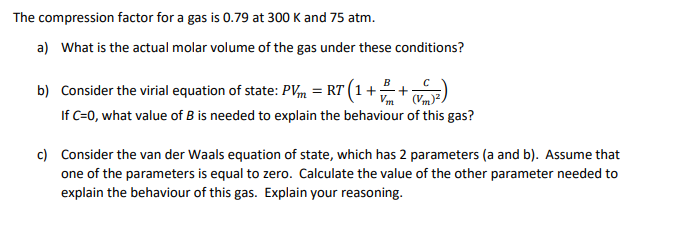
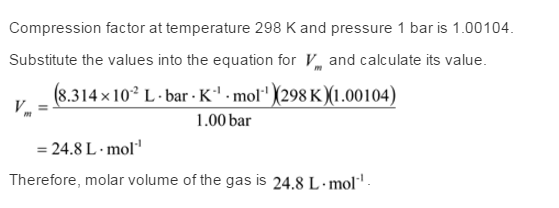
Solved The compression factor (Z) for a real gas can be
The compressibility factor for one mol of a vanderwalls gas at 0

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

Real gasses For an ideal gas, the compressibility factor Z = PV

Which of the following statements is/are correct? (a) all real
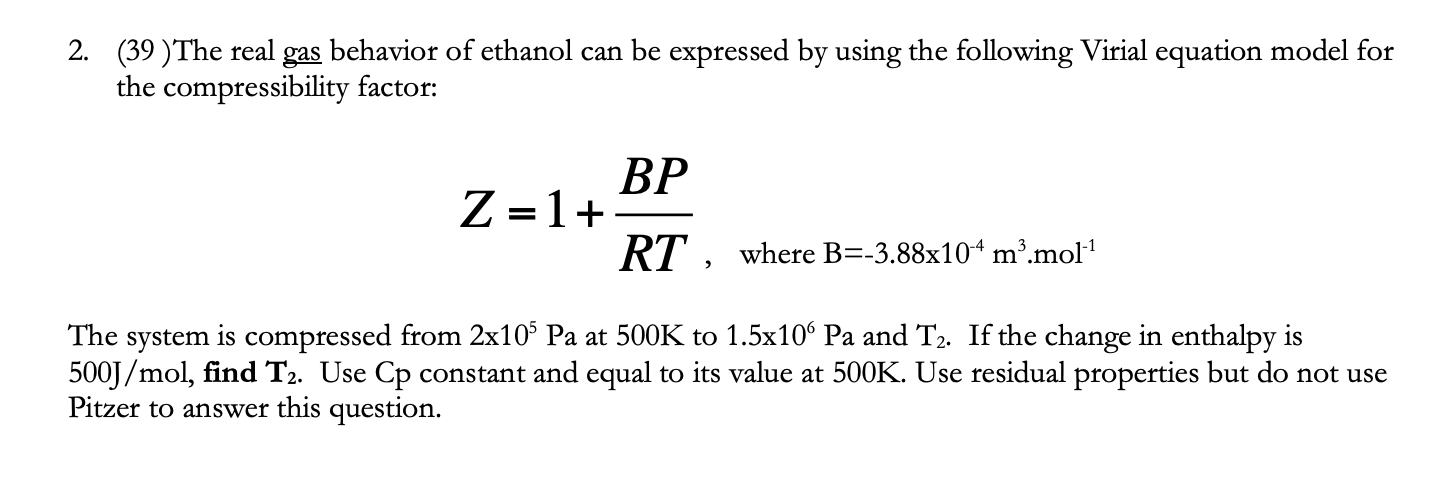
Solved The real gas behavior of ethanol can be expressed by
Is z (compressibility factor) vs P (pressure) graph drawn by

thermodynamics - Variation of compressiblity factor with

the equation of state of a gas is p(v-nb)=rt where b and r are

Compressibility factor - Wikipedia

Odia] The compressibility factor of a gas is defined as Z =(PV)/Nrt.

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Solved The compressibility factor, Z, can be thought of as a

The given graph represents the variation of compressibility factor

At high pressure, the compressibility factor 'Z' is equal toa

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks