Solved] Why is the compressibility factor less than 1 at most conditions?

The compressibility of a gas is than unity STP, therefore:V_{m} > 22.4 LV_{m} < 22.4 LV_{m} = 22.4 LV_{m} = 44.8 L

The value of compressibility factor at the critical state the gas matches with the `Z_(c )` is

Non-Ideal Gas Behavior Chemistry: Atoms First
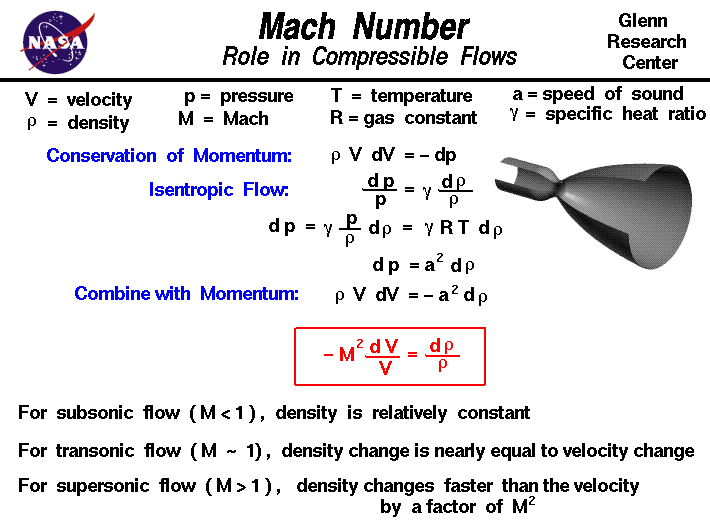
Role of Mach Number in Compressible Flows

The compressibility factor of a gas is less than 1 at STP. Its mola

Physical Chemistry The Compression Factor (Z) [w/1 example]

Real Gases Introductory Chemistry

The compressibility factor Z a low-pressure range of all gases except hydrogen is:Z=(1+ displaystylefrac{a}{V_{m}RT})Z=(1 -displaystylefrac{a}{V_{m}RT})Z=(1+displaystylefrac{Pb}{RT})Z = ( 1 - displaystylefrac{Pb}{RT})

Compressibility factor - Wikipedia
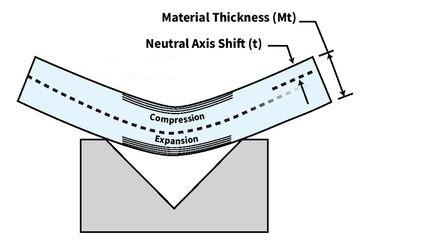
K-factors, Y-factors, and press brake bending precision

Solved Question 2 (1 point) A real gas is maintained under

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange
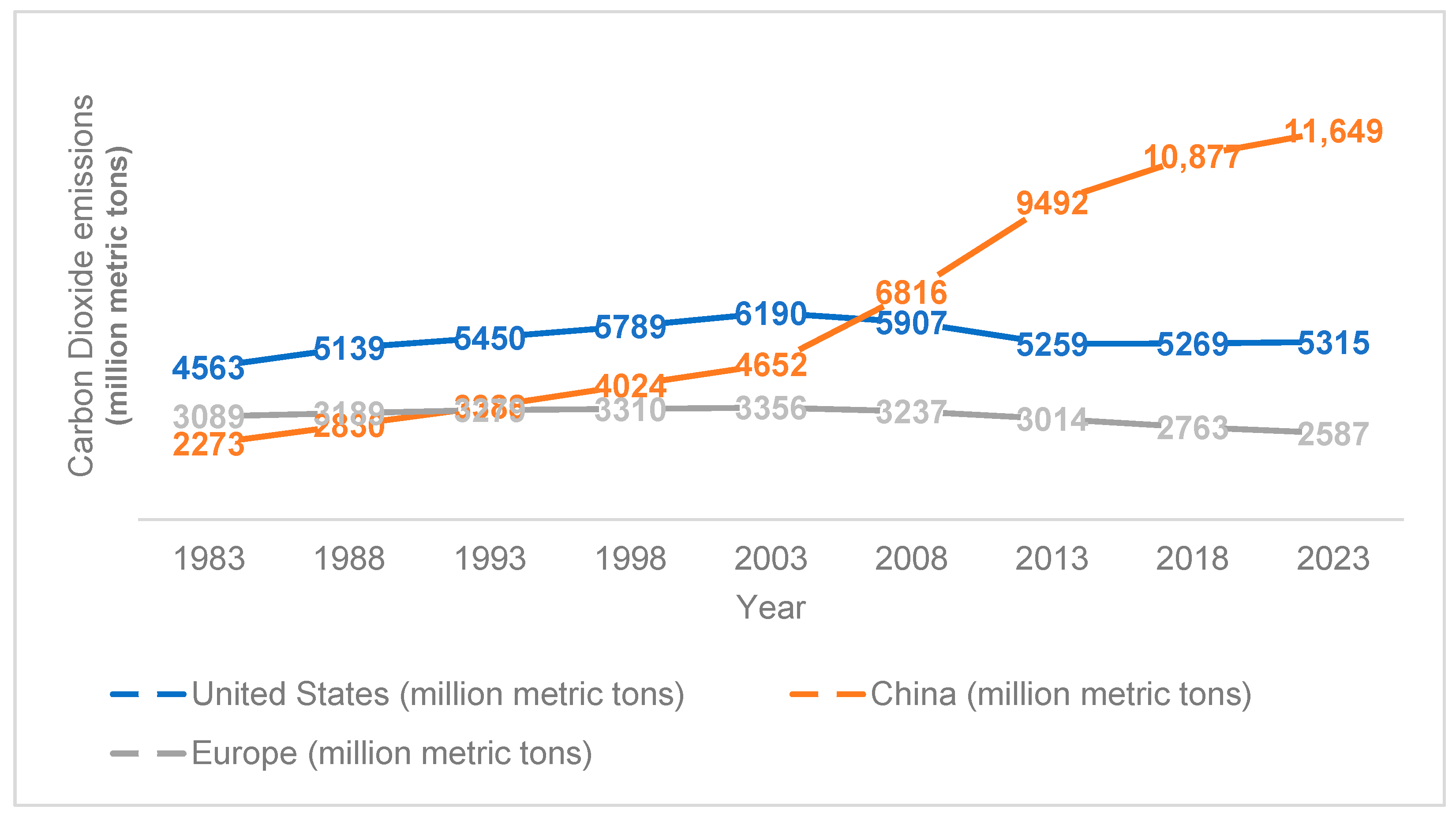
Applied Sciences, Free Full-Text
Why compressibility factor of areal gas is greater than unity at high pressure and temperature? - Quora







