
Gas Stoichiometry: The Ideal Gas Law – Science and Joe
The final tutorial in our series on gas stoichiometry will focus on an equation that combines different theories to comprehensively examine the properties of gases. However, we will soon see why this equation isn’t `ideal’ .
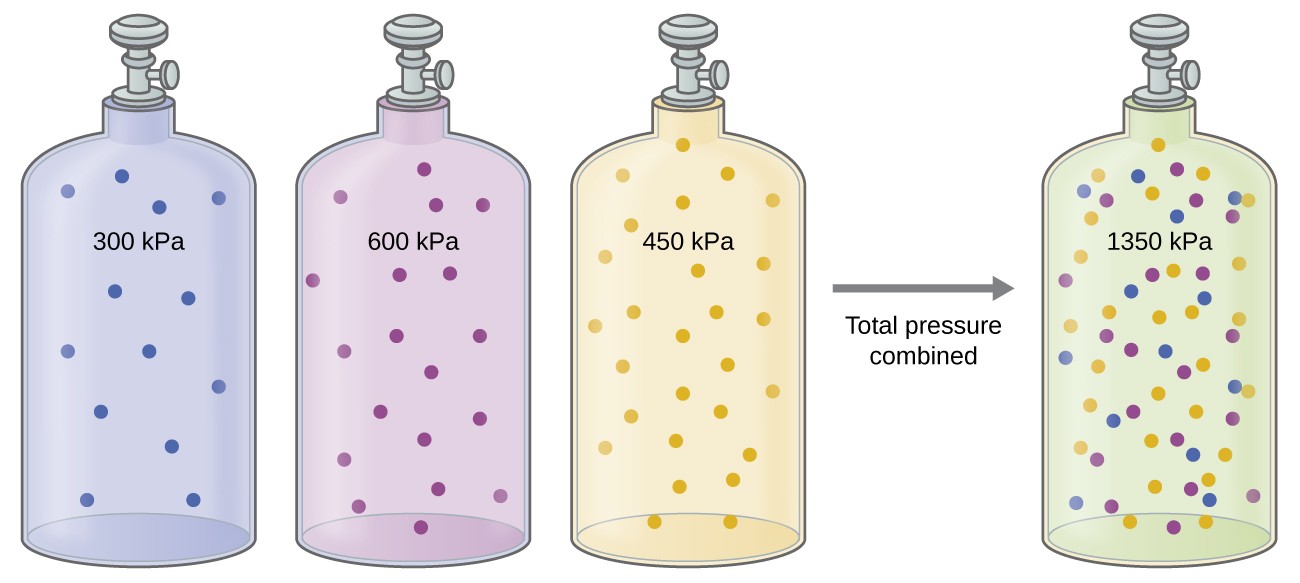
Stoichiometry of Gaseous Substances, Mixtures, and Reactions

Gas Laws - Griger Science
Real-life applications - Gases - Introduction to the Gas Laws

Gay-Lussac's Law - Statement, Formula, Detailed Explanation
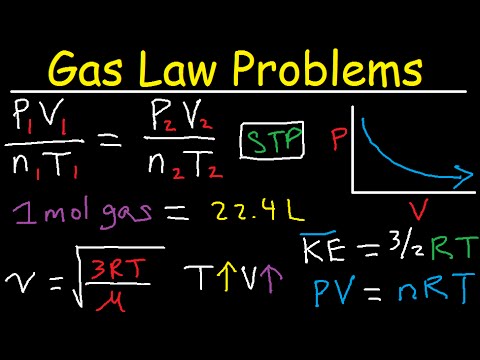
Gas Law Problems Combined & Ideal - Density, Molar Mass, Mole Fraction, Partial Pressure, Effusion

2kemy11 by arta asad - Issuu

i.ytimg.com/vi/FemdpsxtTuU/hq720.jpg?sqp=-oaymwEhC
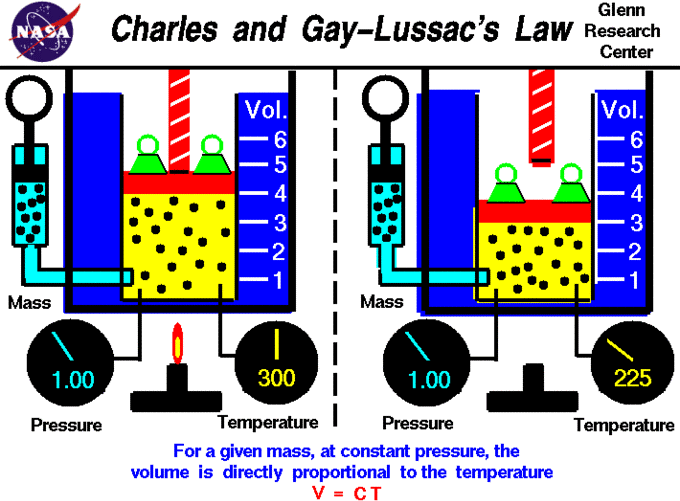
Gas Laws – Introductory Chemistry
How is the Combined Gas Law derived? - Quora

Gas Stoichiometry: The Ideal Gas Law – Science and Joe

Ideal Gas Law - FasterCapital

Gas Law Practice Problems: Boyle's Law, Charles Law, Gay Lussac's, Combined Gas Law; Crash Chemistry
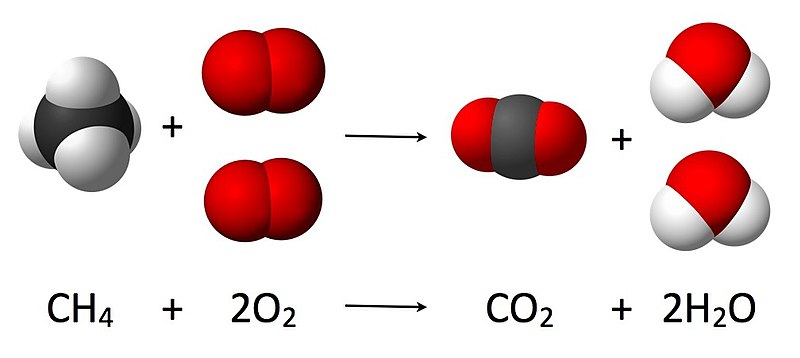
Stoichiometry - Wikipedia

8.3: Relating Pressure, Volume, Amount, and Temperature- The Ideal Gas Law - Chemistry LibreTexts
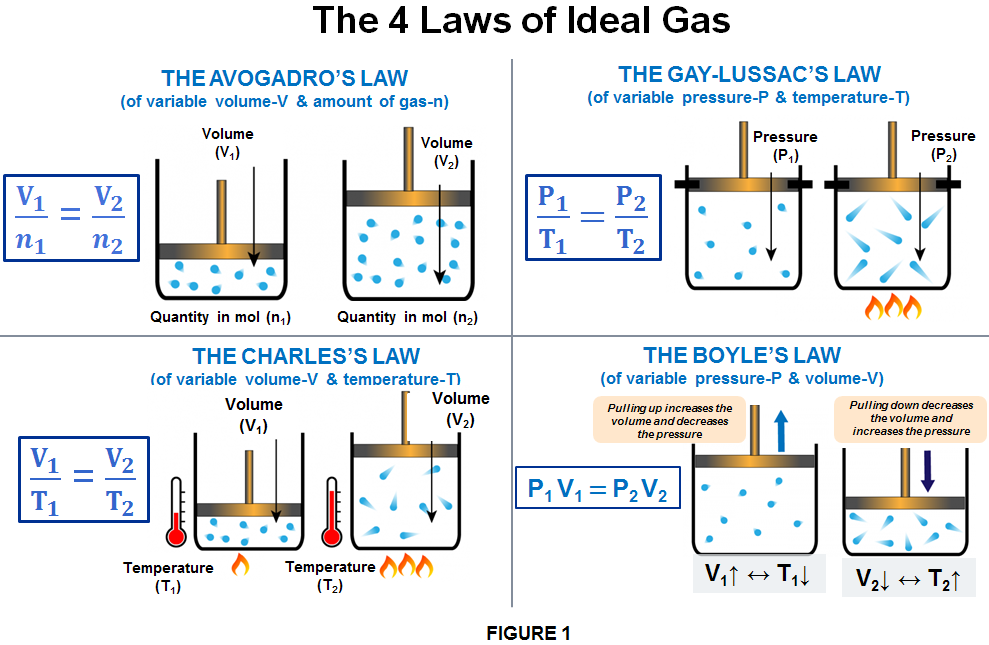

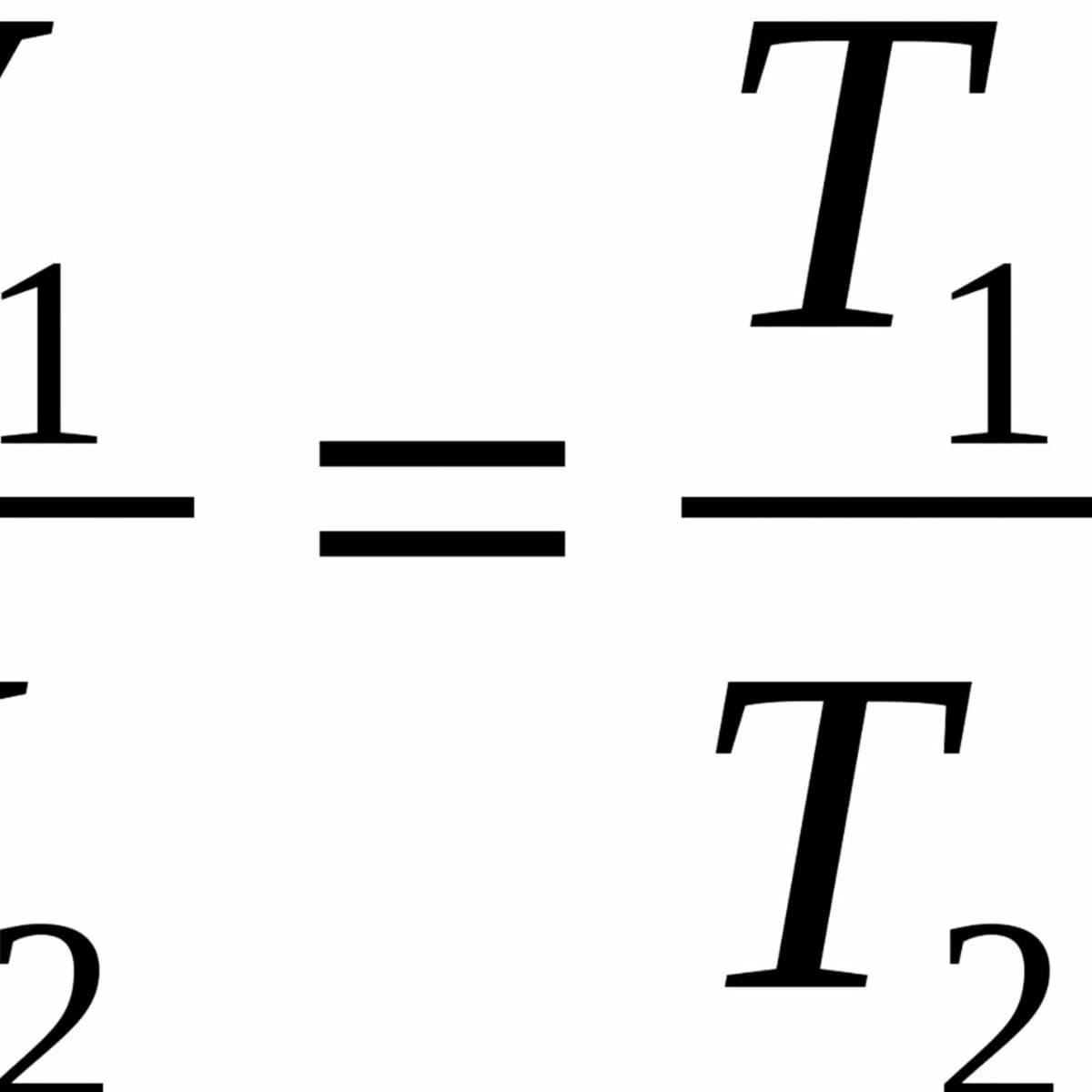
:max_bytes(150000):strip_icc()/200175879-001-56a12e6b5f9b58b7d0bcd67f.jpg)



