The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1)
The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8

Solutions (Colligative Properties, Abnormality in Molar Mass) The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass = 32 g mol -1) in 100 g of CS, (vapour
32. 100 gram of glucose solution has vapour pressure= 40 torr and that of pure liquid is 40.18 torr at a certain temperature. If this solution is cooled to 0.93celsius. What mass
The vapor pressure of a solution having 2 gram of solute X ( gram atomic mass =32 gram/mol ) in 100 gram of CS_2 ( vapour pressure = 854 torr ) is
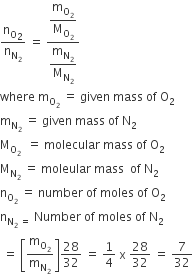
The vapour pressure of acetone at 20oC is 185 torr. When 1.2 g o
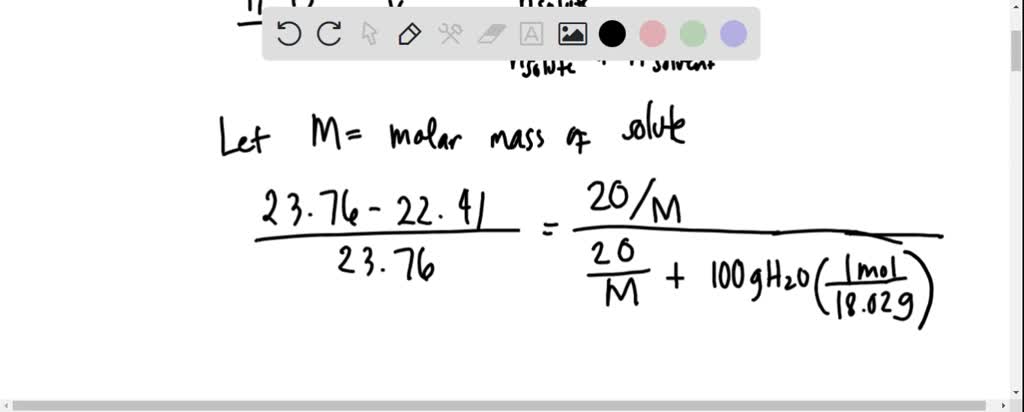
SOLVED: 20 g of solute was added to 100 g of water at 25^oC . The vapour pressure of water and that of solution were 23.76 mmHg and 22.41 mmHg respectively at

Chapter-10 Solutions.pdf - Chemistry - Notes - Teachmint

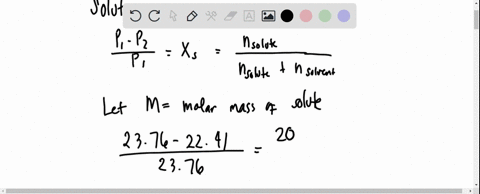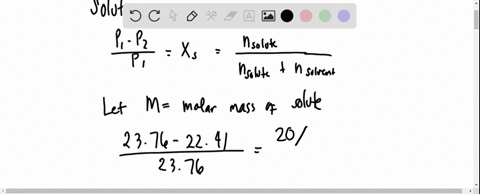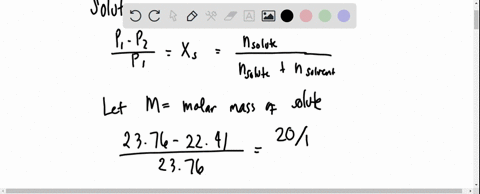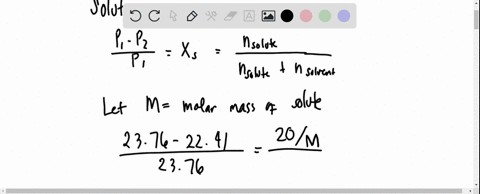
SOLVED: The vapour pressure of a solution having 2.0 g of solute X

PDF) comprehensive physical chemistry
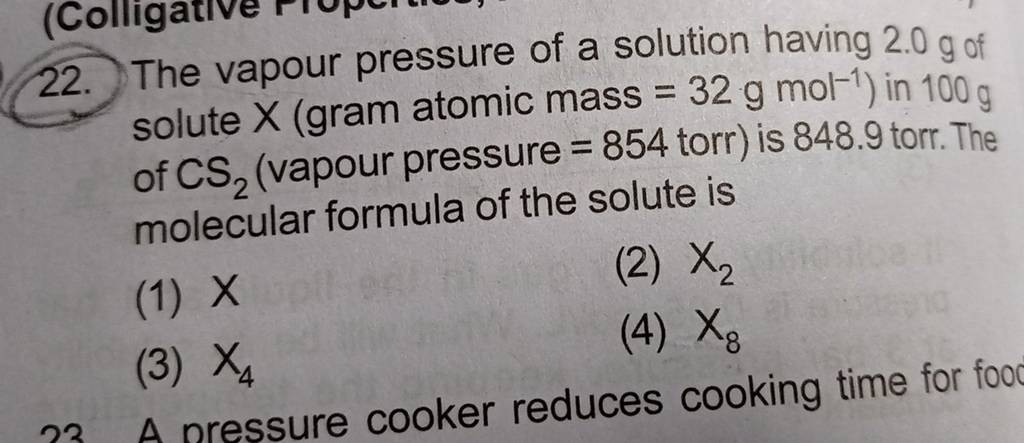
The vapour pressure of a solution having 2.0 g of a solute X( molar mass ..

The vapour pressure of a solution having 2.0 g of a solute X( molar mass ..