
The compressibility factor Z a low-pressure range of all gases
Click here:point_up_2:to get an answer to your question :writing_hand:the compressibility factor z at a lowpressure range of all gases except hydrogen is
Click here👆to get an answer to your question ✍️ The compressibility factor Z a low-pressure range of all gases except hydrogen is-Z-1- displaystylefrac-a-V-m-RT-Z-1-displaystylefrac-a-V-m-RT-Z-1-displaystylefrac-Pb-RT-Z - - 1 - displaystylefrac-Pb-RT-
The van der Waals equation for real gases is -P-aVm2-Vm-x2212-b-RT

2. U 0.52, 0.68, 0.74 At low pressure, the comprensibility factor is given as (1) - RTV Pb RT 12 12 Photo (3) 1+ TV Pb RT 3. 10 mole of an ideal gas 27°C ernands

Consider the graph between compressibility factor Z and pressure P The correct increasing order of ease of liquefaction of the gases shown in the above graph is

Compressibility factor - Wikipedia

Real gas z-factor, as attributed to Standing and Katz, 9 plotted as a
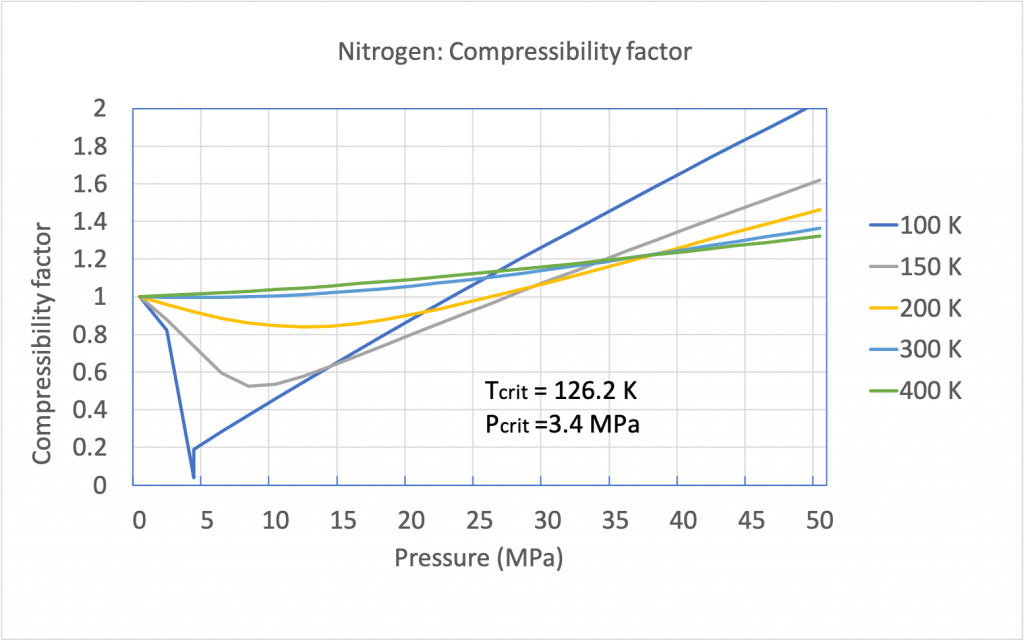
3.3: Real gas and compressibility factor - Engineering LibreTexts

Objectives_template

physical chemistry - Is the compressibility factor smaller or greater than 1 at low temperature and high pressure? - Chemistry Stack Exchange

Compressibility Factor (Z-Factor) Equation of State

Plot of experimental measurements of the z-factor
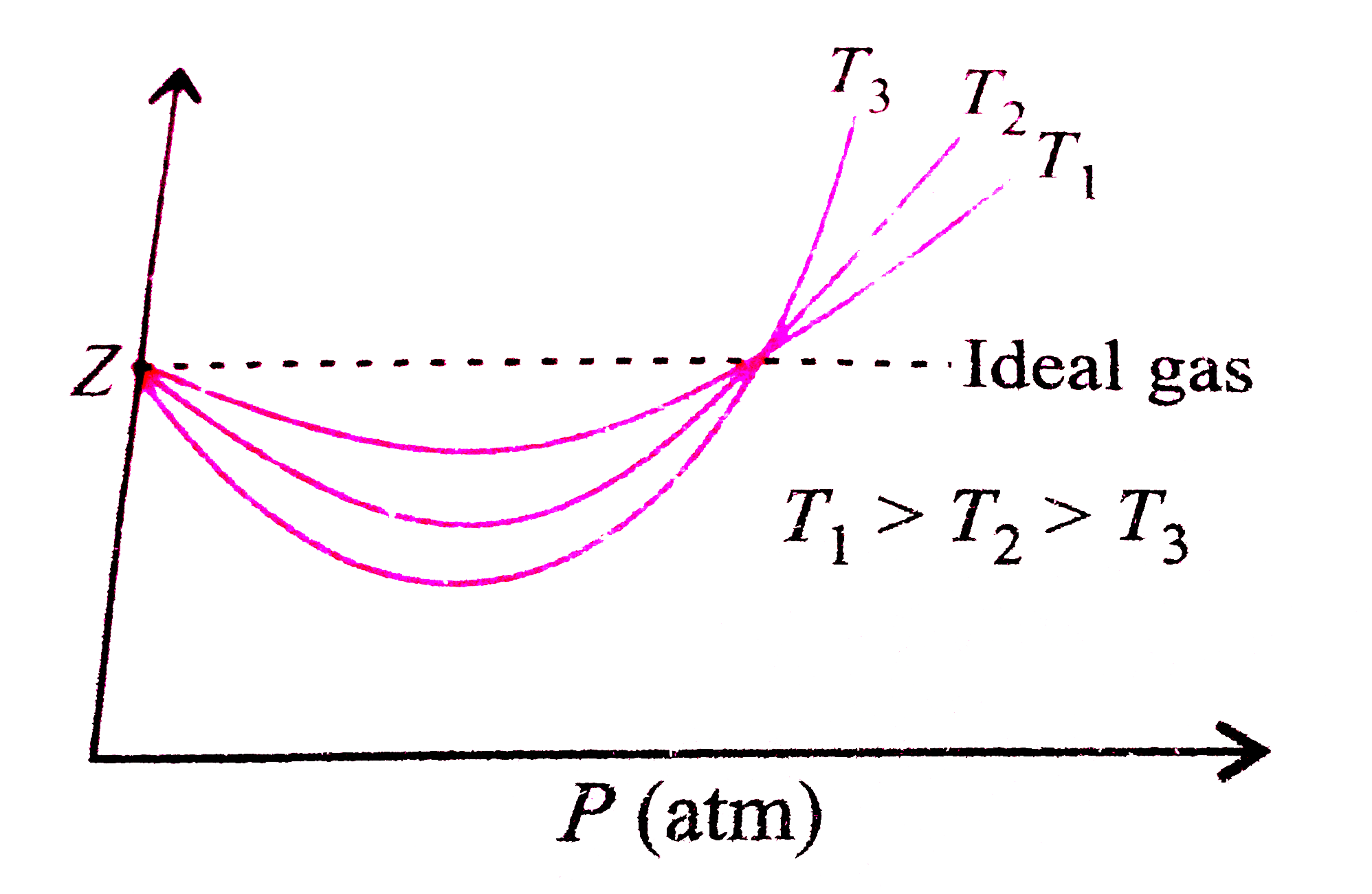
At intermediate pressures , most gases show Z lt 1.

09 DEFINITION Behaviour of gases by van der Waals equation (P+*}(0-b) = RT may be written as (P+*}() =RT of PV + 9 =RT of PV=RT - For large V (at very

Compressibility Factor Charts - Wolfram Demonstrations Project









