At high pressure, the compressibility factor 'Z' is equal toa)unityb) c) d)ZeroCorrect answer is option 'C'. Can you explain this answer? - EduRev NEET Question

For compressibility factor, Z, which of the following is /are correct?
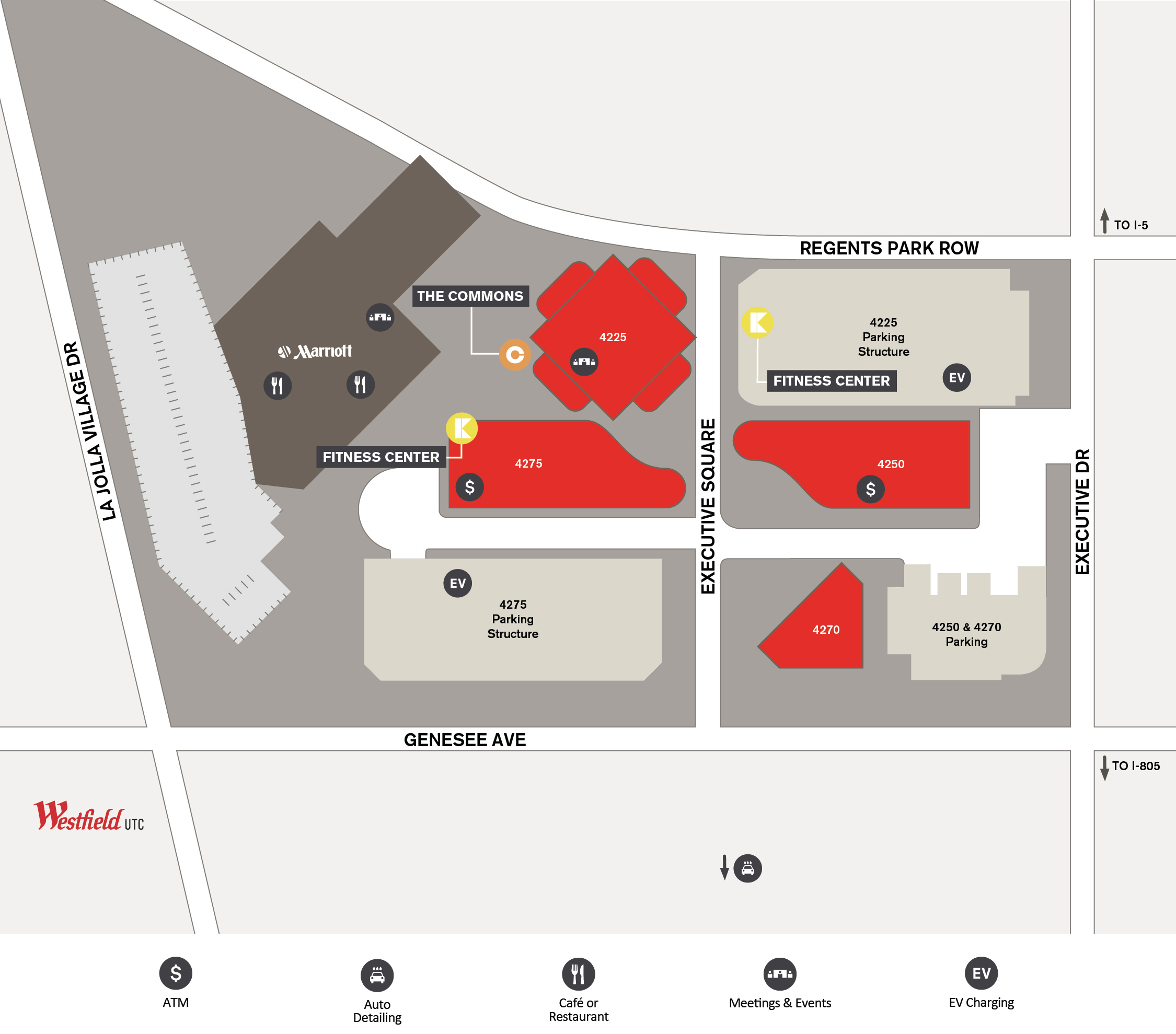
Solved Exercise 4.7: Shown below are compressibility data

THREE STATES OF MATTER General Properties of Gases. - ppt download

The compressibility factor Z a low-pressure range of all gases except hydrogen is:Z=(1+ displaystylefrac{a}{V_{m}RT})Z =(1-displaystylefrac{a}{V_{m}RT})Z=(1+displaystylefrac{Pb}{RT})Z = ( 1 - displaystylefrac{Pb}{RT})

At high pressure, the compressibility factor 'Z' is equal toa)unityb) c) d)ZeroCorrect answer is option 'C'. Can you explain this answer? - EduRev NEET Question
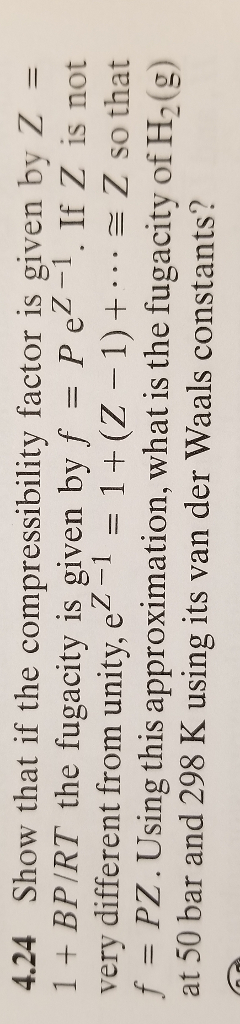
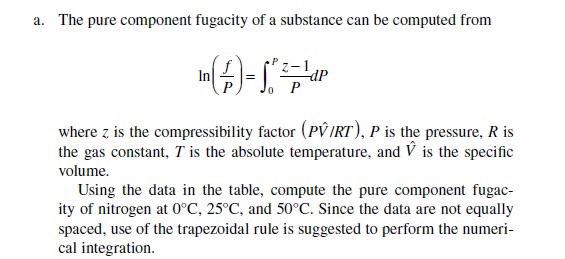
Solved 4.24 Show that if the compressibility factor is given
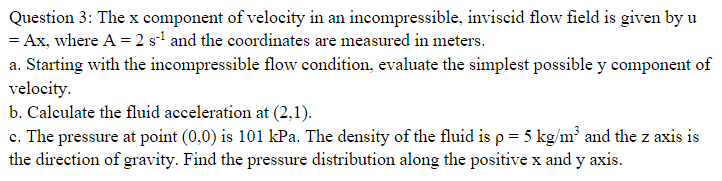
Solved Question 3: The x component of velocity in an
NEET Practice Test - 11 Free MCQ Practice Test with Solutions - NEET

The compressibility factor Z of a gas is less than unity at STP. Therefore
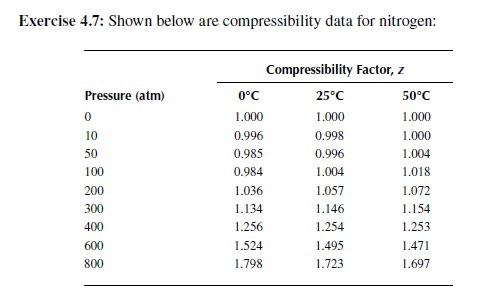
Solved Exercise 4.7: Shown below are compressibility data

Solved 3.91. The definition of compressibility factor Z, Eq.