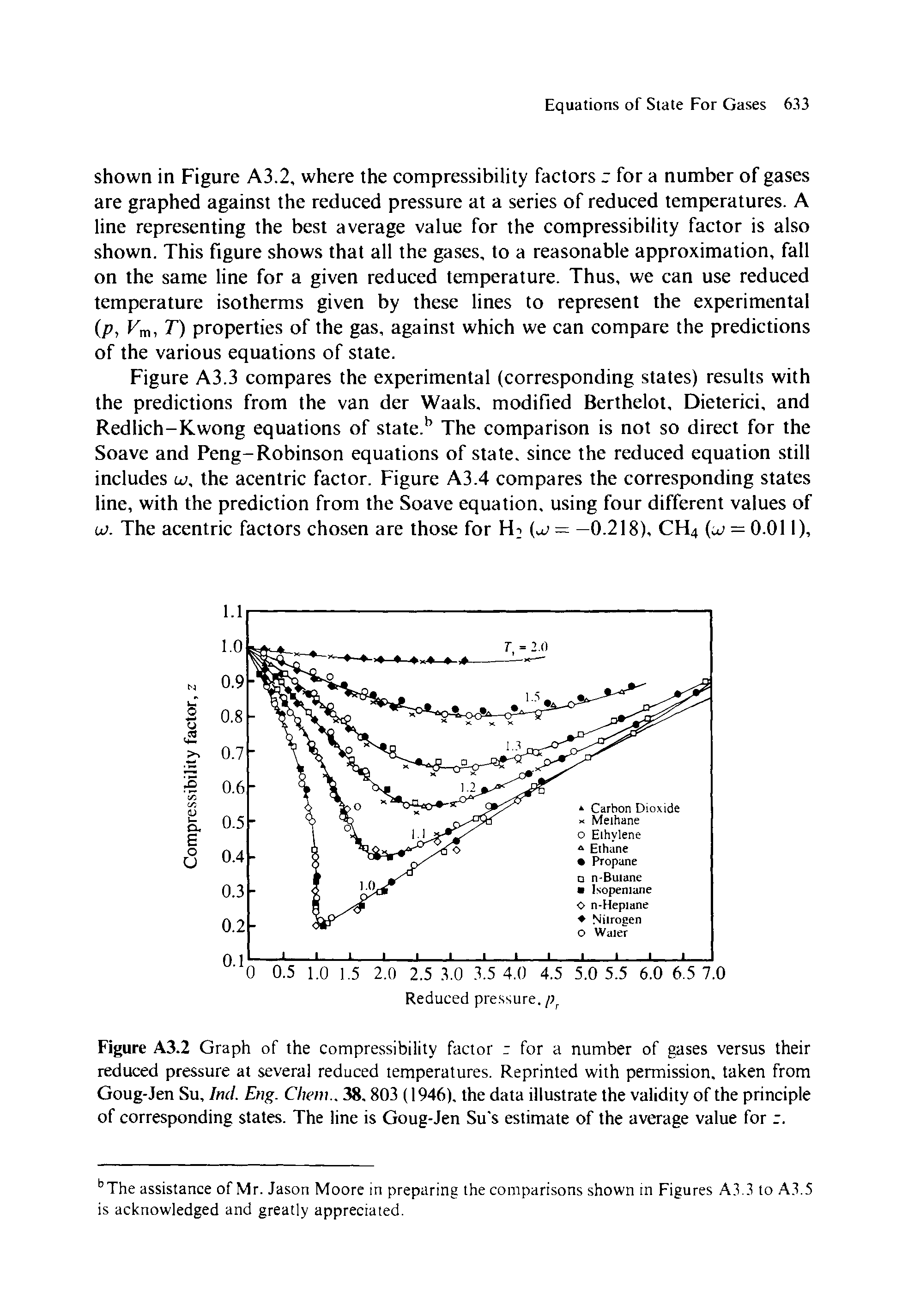
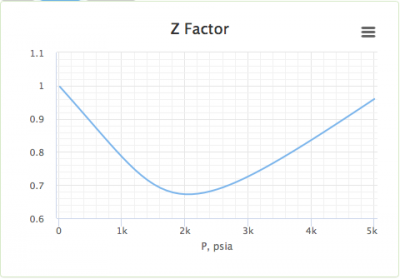
The graph of compressibility factor (Z) vs. P for one mole of a real gas ..
Solution For The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following diagram. The graph is plotted at constant temperature 273K. If the slope of graph at very hi
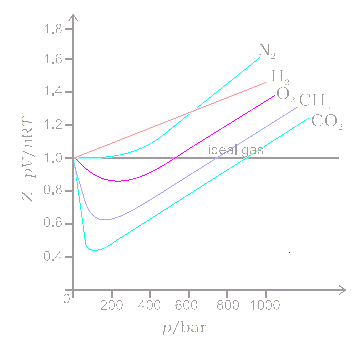
The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following diagram. The graph is plotted at constant temperature 273K. If the slope of graph at very high pressure (dPdZ) is (2.81)atm−1 , then calculate volume of one mole of real gas molecules (in L/mol) Given : NA=6×1023 and R=27322.4LatmK−1mol−1

What is compressibility factor? What is its value for ideal gas
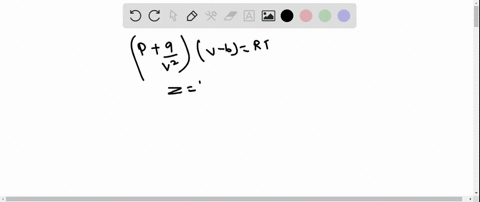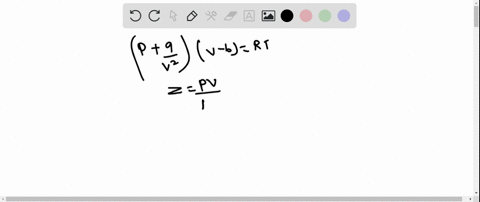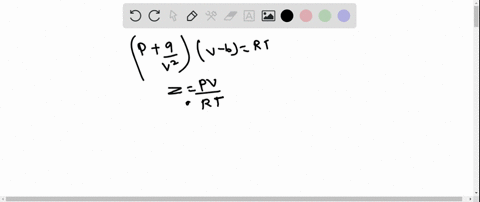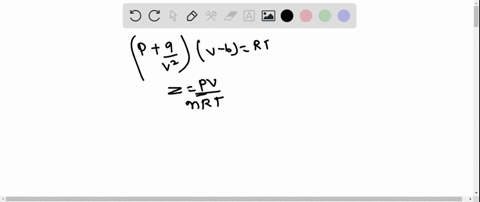
⏩SOLVED:The behaviour of a real gas is usually depicted by

Gaseous State, PDF, Gases
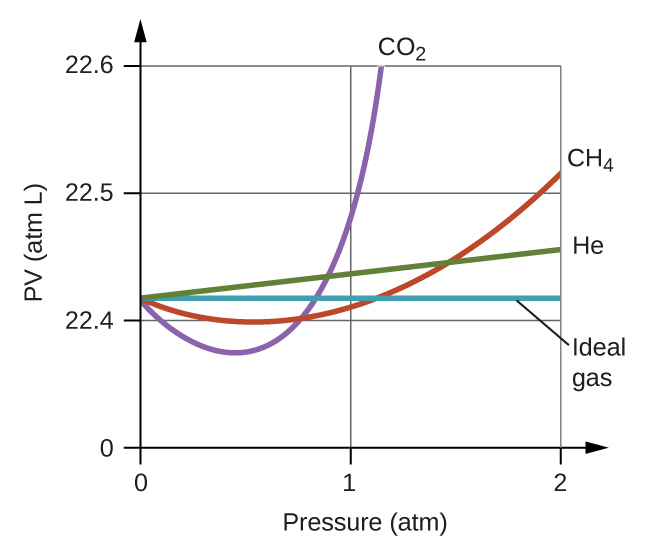
Non-Ideal Gas Behavior – Atoms First / OpenStax
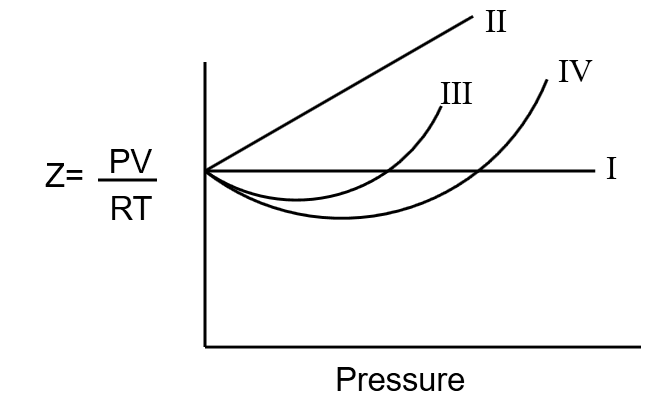
For the nonzero volume of molecules having no forces of attraction

Gaseous State, PDF, Gases

Gaseous State, PDF, Gases
In the following compressibility factor (Z) vs pressure graph at

Compressibility factor - Wikipedia
Van der waals equation: Derivation, Explanation

Gaseous State, PDF, Gases

The graph of compressibility factor (Z) vs. P for one mole of a