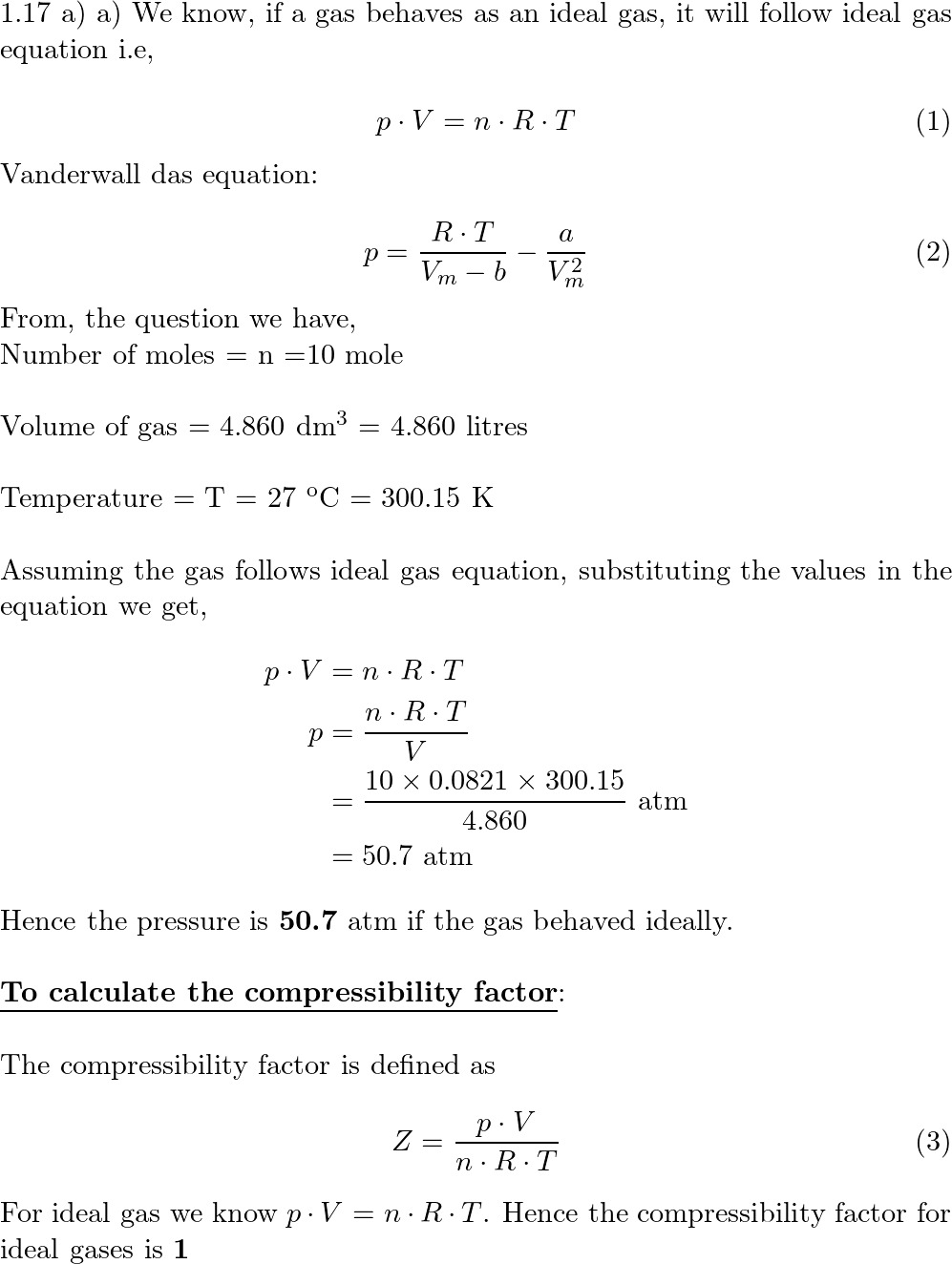
a) Suppose that $10.0\ \mathrm{mol}\ \mathrm{C}_{2} \mathrm
4.5
(343)
Write Review
More
$ 15.50
In stock
Description

The density of an aqueous solution containing $10.0$ percent

Chapter 10, Balances on Transient Processes Video Solutions

Lecture 2 Fundamentals of Data, Information, and Knowledge

Solved The rate constant for the decomposition of a certain

⏩SOLVED:At a certain temperature, Kc=0.18 for the equilibrium

Refer to the bromine phase diagram you sketched in Problem 1

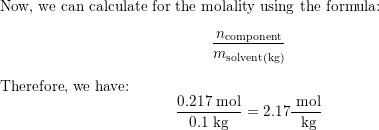

The density of an aqueous solution containing $10.0$ percent
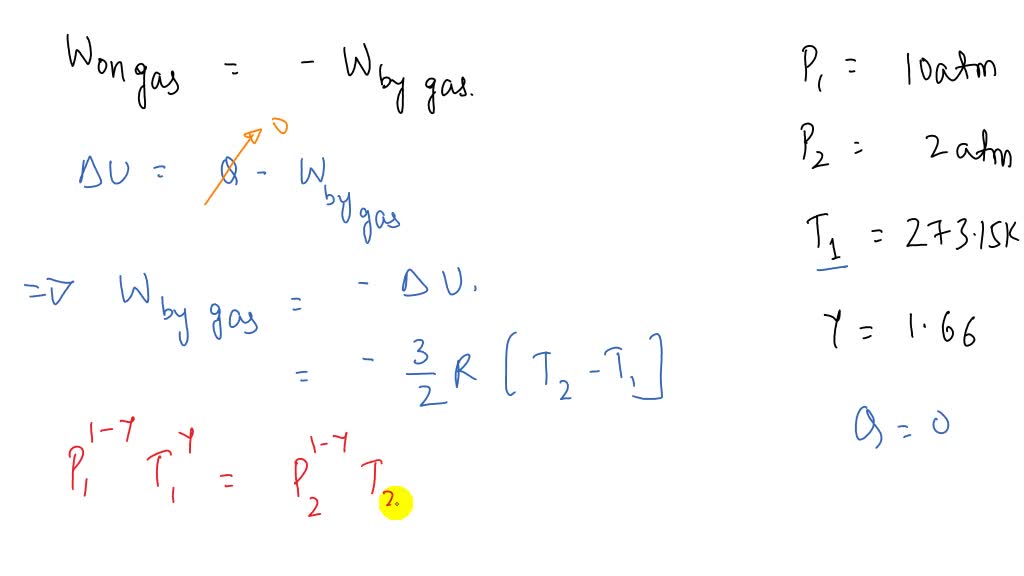
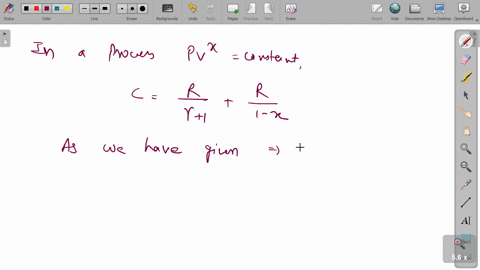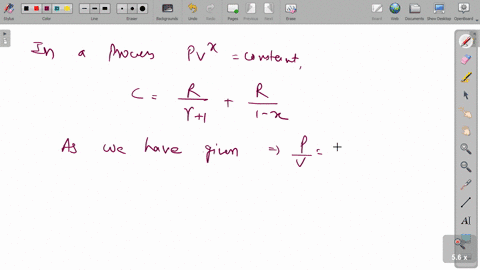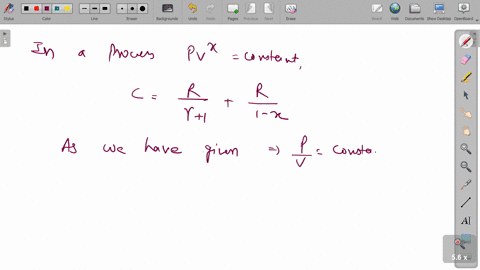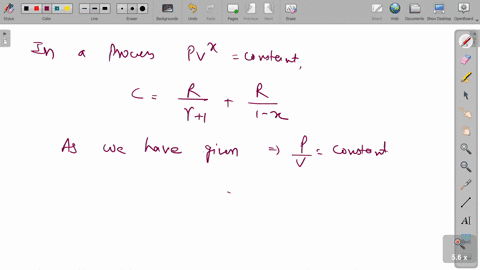
⏩SOLVED:One mole of an ideal monatomic gas (y=1.66),(γ=1.66
⏩SOLVED:A cylinder of volume 0.300 m^3 contains 10.0 mol of neon
Chapter 16, Solubility and Complex Ion Equilibria Video Solutions
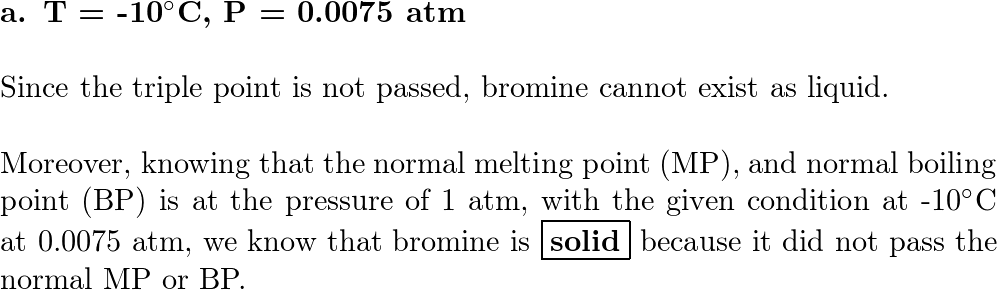
Refer to the bromine phase diagram you sketched in Problem 1
You may also like