200 g of a sample of limestone liberates 66 g of CO2 on heating
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

4.64 A sample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate

PDF) Sequestration of Carbon Dioxide in Coal with Enhanced Coalbed Methane RecoveryA Review †
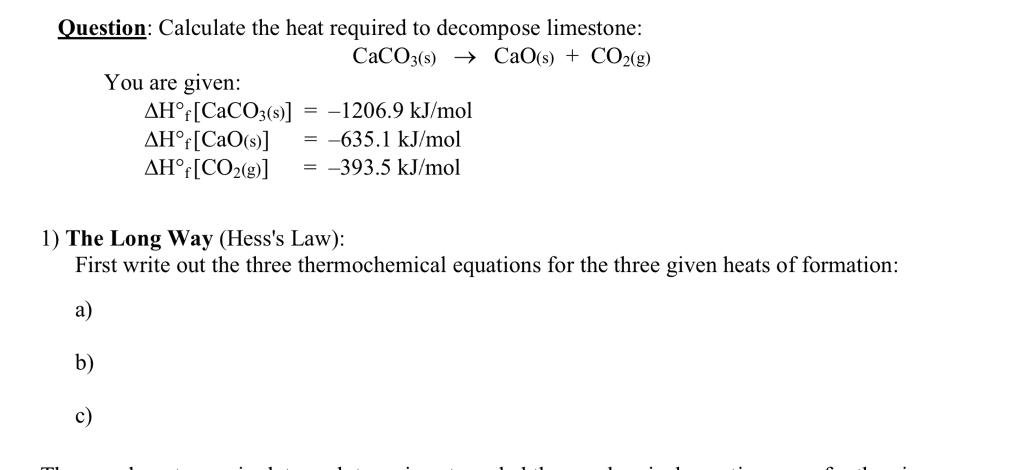
Solved Question: Calculate the heat required to decompose

Topical Mock Chemistry Questions, PDF, Chemical Elements

Thermochemical energy storage system development utilising limestone - ScienceDirect
200g of a sample of limestone liberates 66 g of co2 on heating. The

Solved 6 Calcium carbonate (limestone) decomposes when
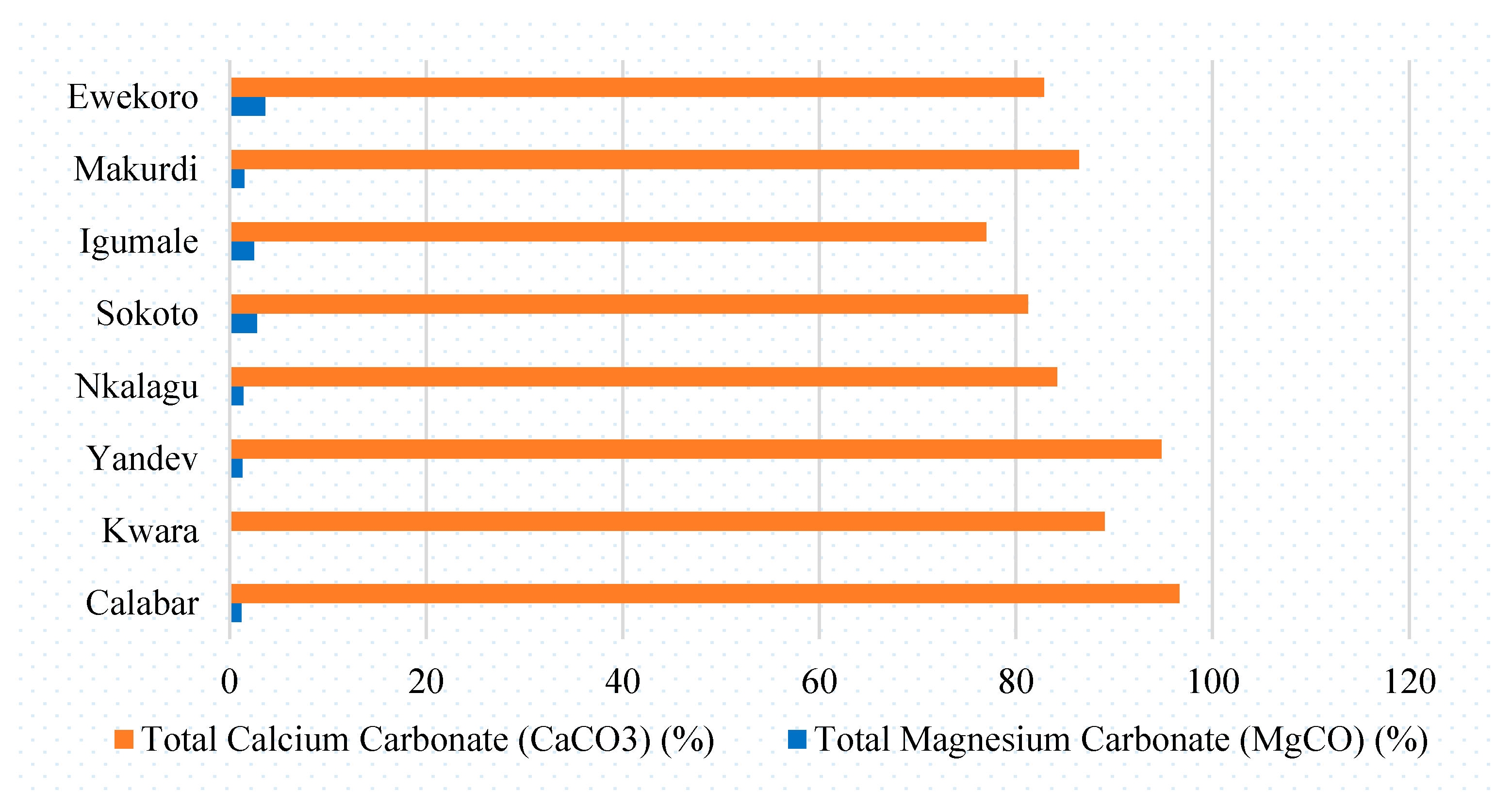
Atmosphere, Free Full-Text

0958 ch11.pdf - Index of - Free
200g of a sample of limestone libetates 66g of CO2 on heating.The percentage impurity of CaCo3in the limestone is 1) 95









