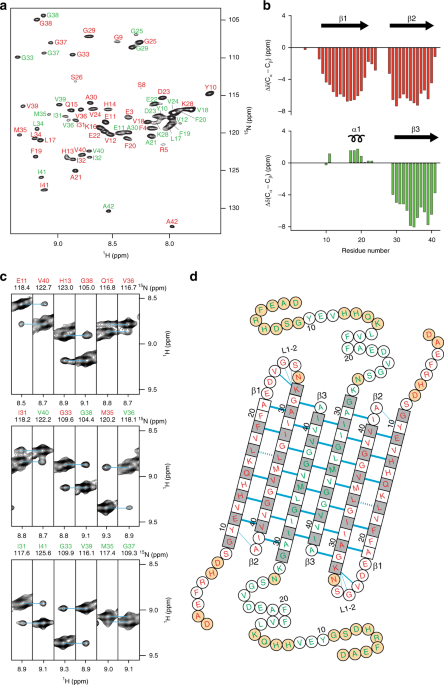
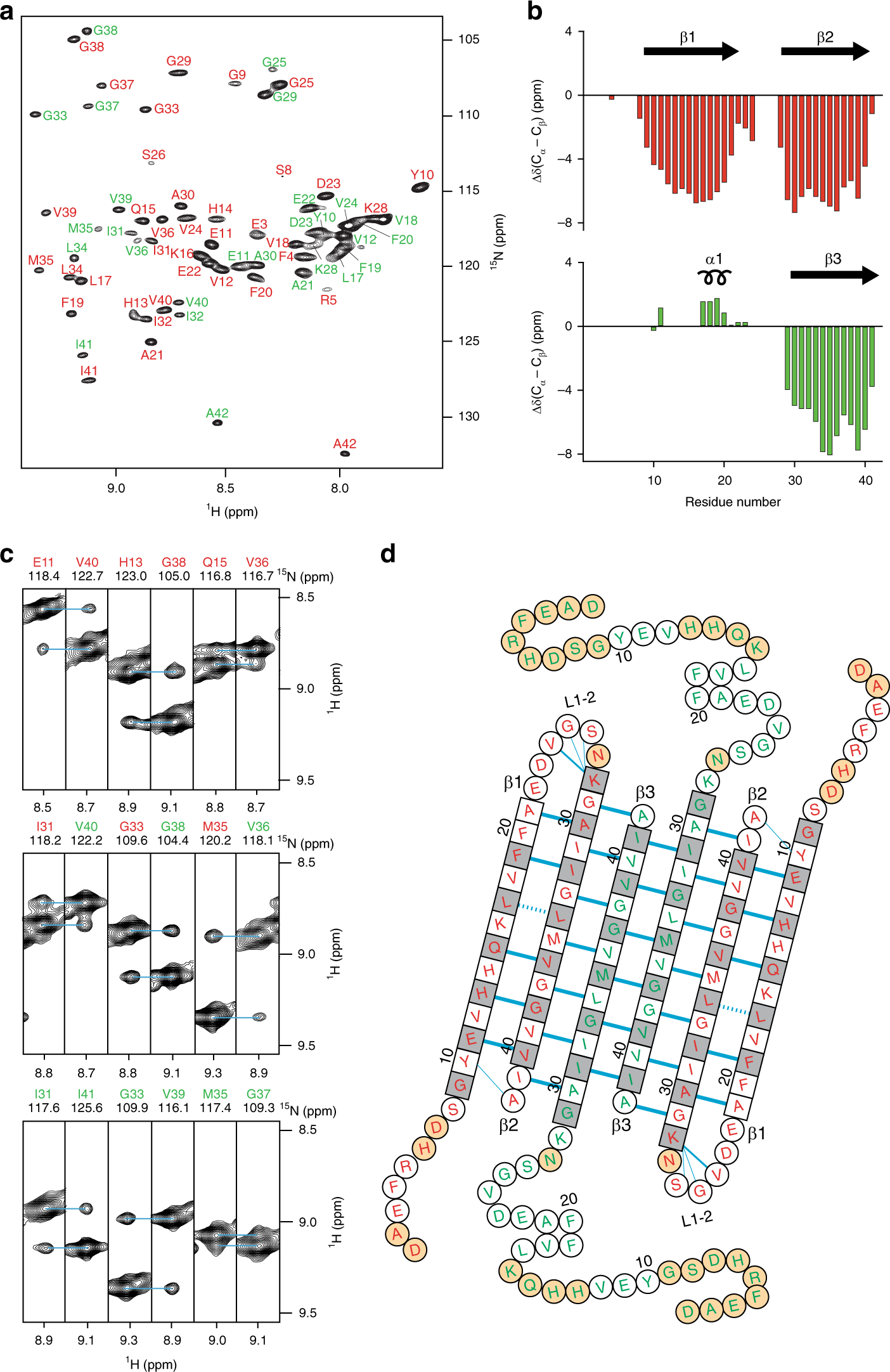
Aβ(1-42) tetramer and octamer structures reveal edge conductivity pores as a mechanism for membrane damage
4.7
(219)
Write Review
More
$ 10.00
In stock
Description
Aβ(1-42) tetramer and octamer structures reveal edge conductivity

PDF) Cell-Penetrating Peptides with Unexpected Anti-Amyloid
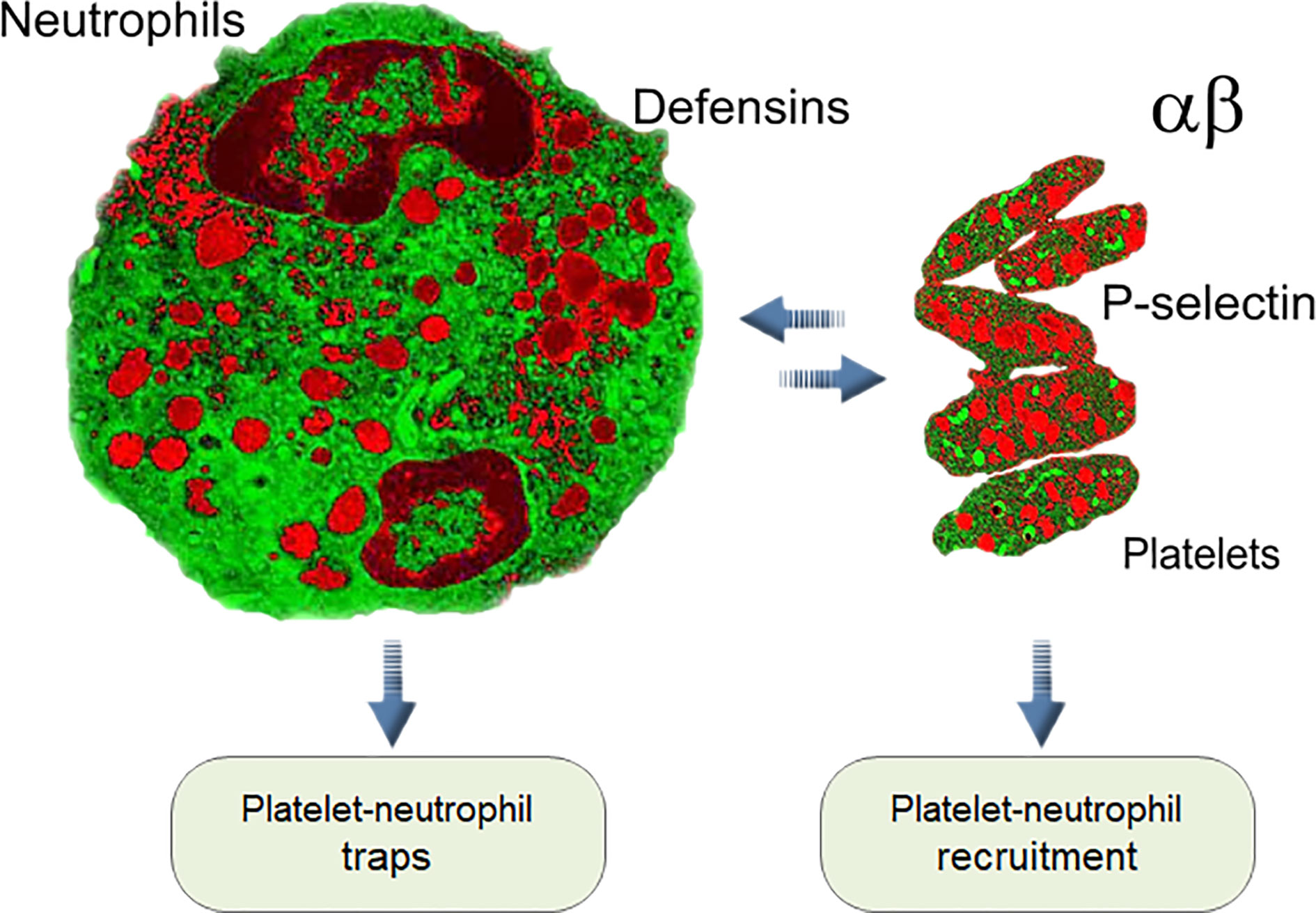
Frontiers On the Role of Platelet-Generated Amyloid Beta
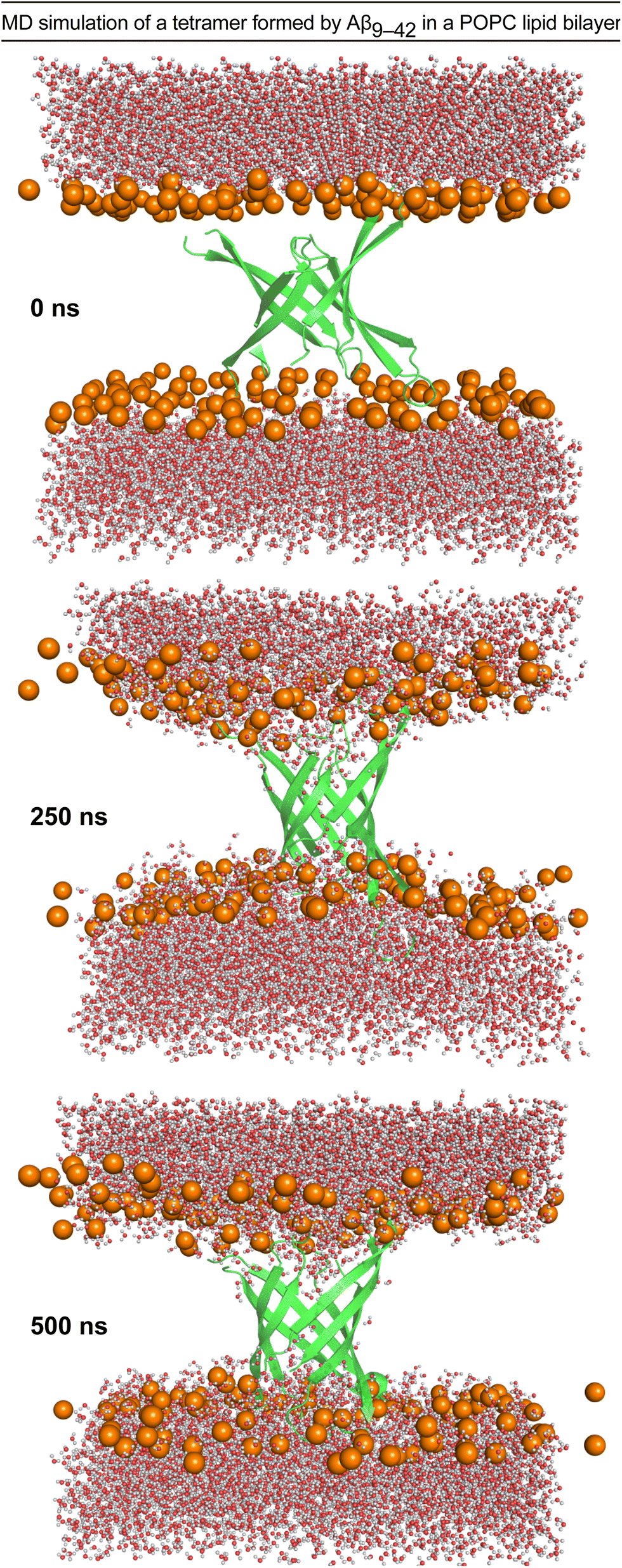
A β-barrel-like tetramer formed by a β-hairpin derived from Aβ
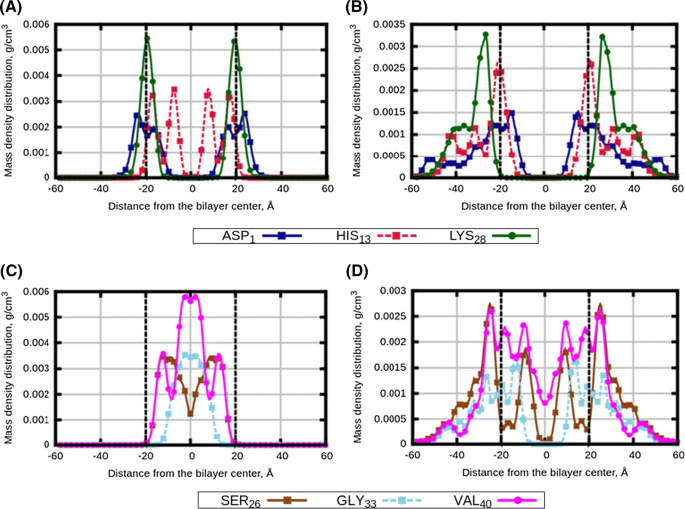
Effect of lipid saturation on amyloid-beta peptide partitioning

βPFOAβ(1-42) samples can be enriched in either tetramers or
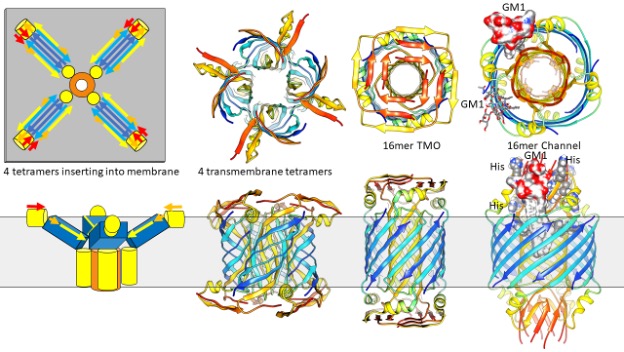
Why are the root causes of amyloid-associated diseases so

Effects of the Hydrophilic N-Terminal Region on Aβ-Mediated

PDB 6rhy structure summary ‹ Protein Data Bank in Europe (PDBe

RCSB PDB - 6RHY: Structure of pore-forming amyloid-beta tetramers

Structural architecture of amyloid-β oligomers, curvilinear
Related products
You may also like