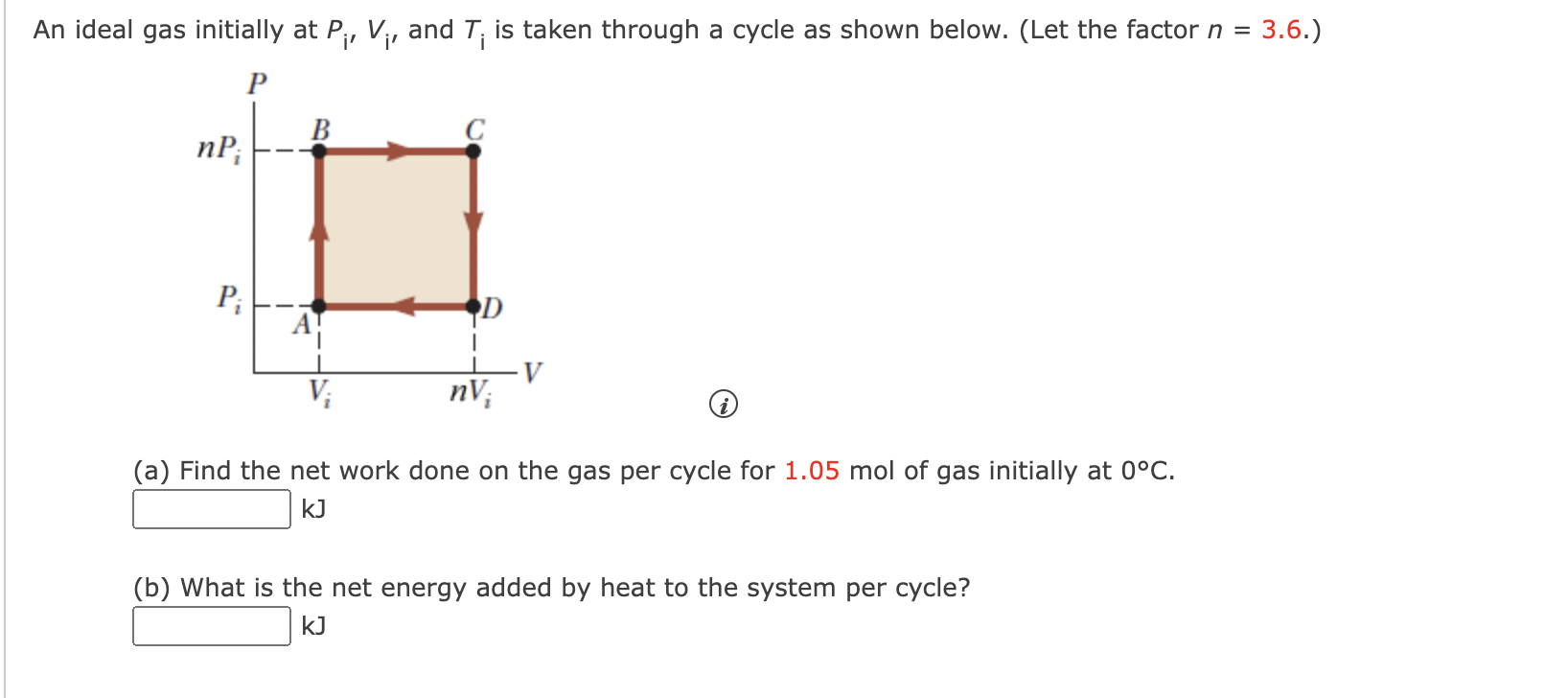
Solved An ideal gas initially at Pi, V;, and T; is taken
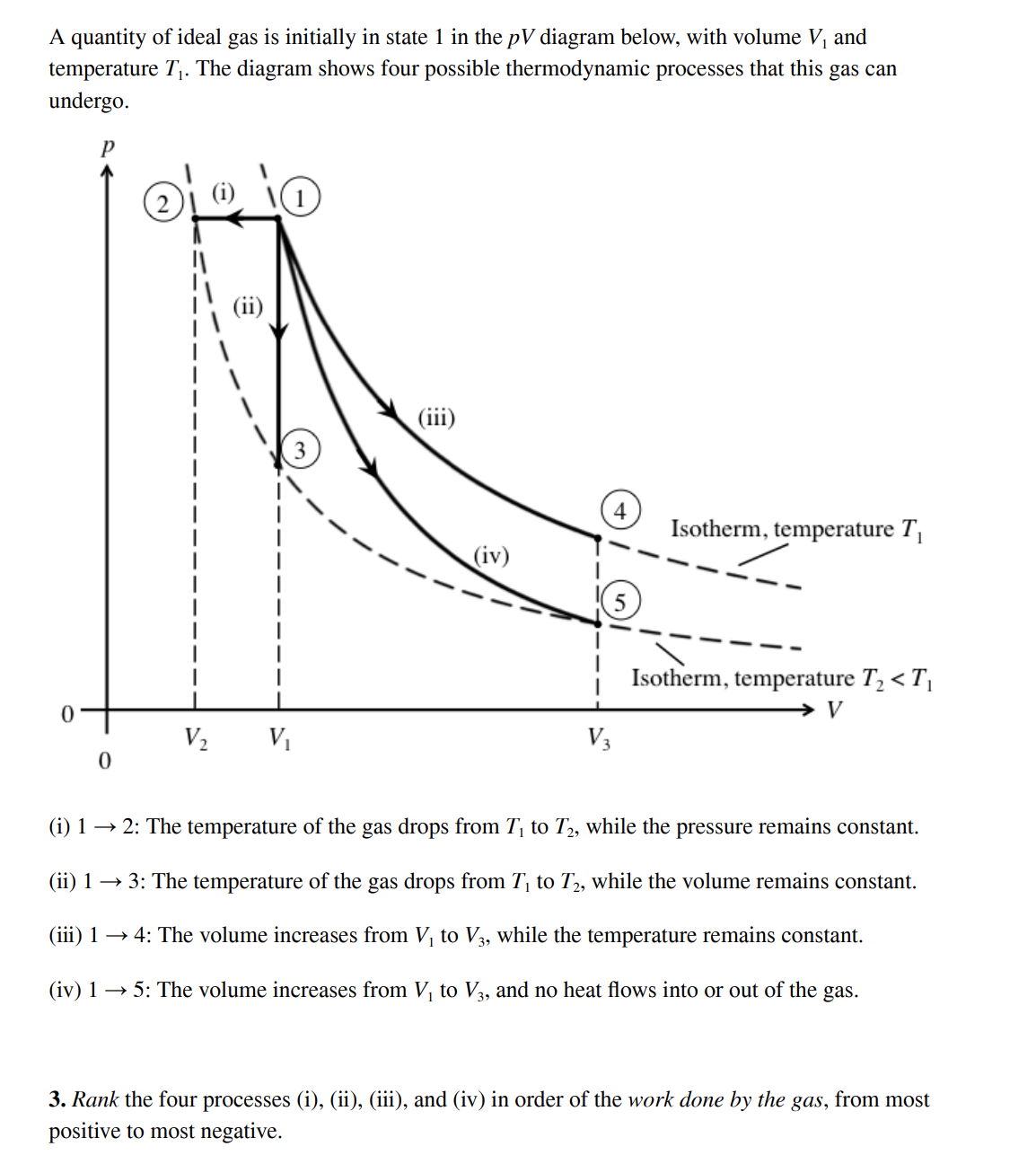
Solved A quantity of ideal gas is initially in state 1 in

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
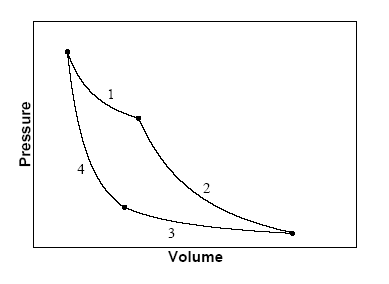
entropy

What is the Maxwell-Boltzmann distribution? (article)

An ideal gas initially at a state (P1,V1) is allowed to expand isothermally to a state (P2, V2).

An ideal gas has initial volume V and pressure p. If the volume of gas is doubled during expansion, then minimum work will be done in which thermodynamic process ?A. Isobaric processB.

An ideal gas initially P_i ,V_i , and T_i is taken through a cycle as shown in Figure. (a) Find the net work done on the gas per cycle 1.00 mol of
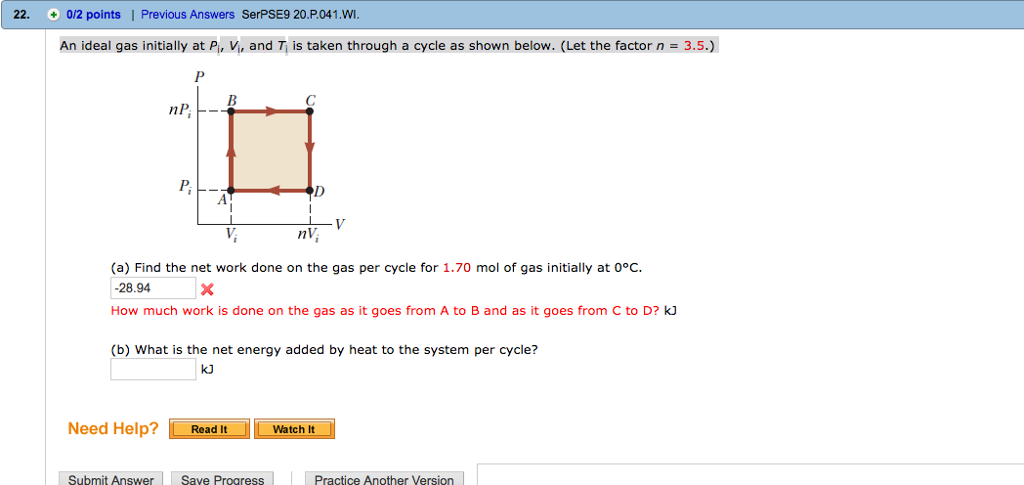
Solved An ideal gas initially at Pi, Vi, and Ti is taken

An ideal gas is enclosed in a cylinder with a movable piston on top of it. The piston has a mass of
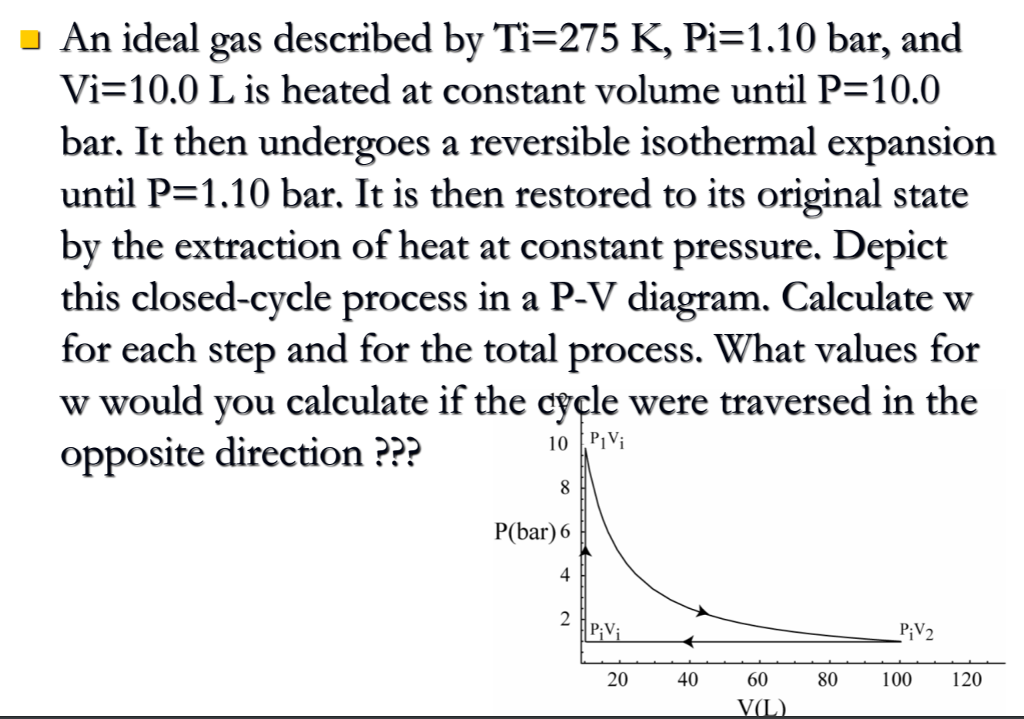
Solved - An ideal gas described by Ti-275 K, Pi-1.10 bar









