
What is the change in internal energy (in J) of a system that absorbs 0.464 kJ of heat from its surroundings and has 0.630 kcal of work done on it?
4.8
(283)
Write Review
More
$ 15.99
In stock
Description
I found an increase of 3100J Have a look

Irrigation and Drainage Engineering 9783319056999, 3319056999

15.4 What is the change in internal energy of a system which

SOLVED: What is the change in internal energy of a system if the

HVAC Engineer's Handbook, Eleventh Edition [11 ed
How to calculate ΔE when the system absorbs 250 J of heat energy

How to calculate ΔE when the system absorbs 250 J of heat energy
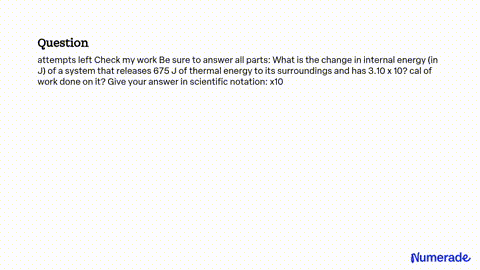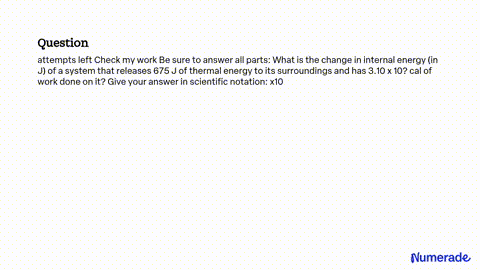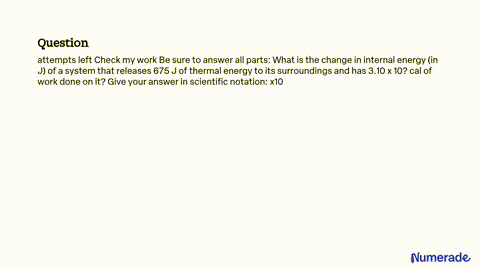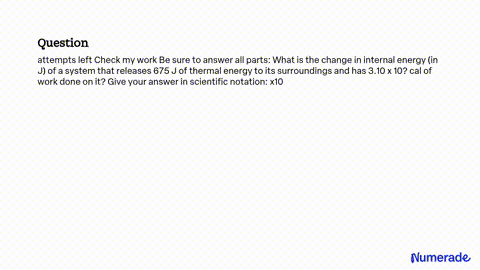
SOLVED: attempts left Check my work Be sure to answer all parts

chemia - Studia

Solved: Chapter 6 Problem 11P Solution
PDF) Resilient and Sensitive Key Points of the Photosynthetic
Related products
You may also like








