The compressibility factor Z for an ideal gas will be
The compressibility factor Z for an ideal gas will be

Compressibility factor (gases) - Citizendium

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks

Compressibility Factor - Thermodynamics I, EGN 3343, Study notes Thermodynamics
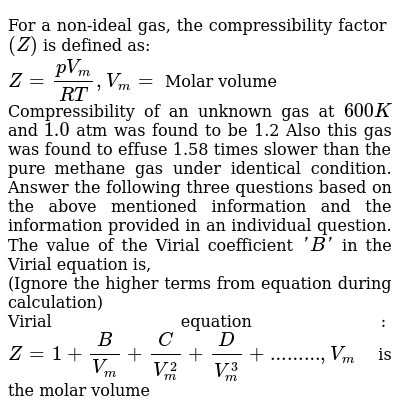
For a non-ideal gas, the compressibility factor (Z) is defined as: Z

Real Gases vs Ideal Gases & the Compressibility Factor

Marathi] The compressibility factor of a gas is defined as z = PV //R

Question ( 13 quad ) QnDirections: The answer to the following

For a non-ideal gas, the compressibility factor (Z) is defined as: Z

Compressibility factor - Wikipedia

Objectives_template

If a gas gets half compressed, compared to an ideal gas, the compressibility factor Z is equal to
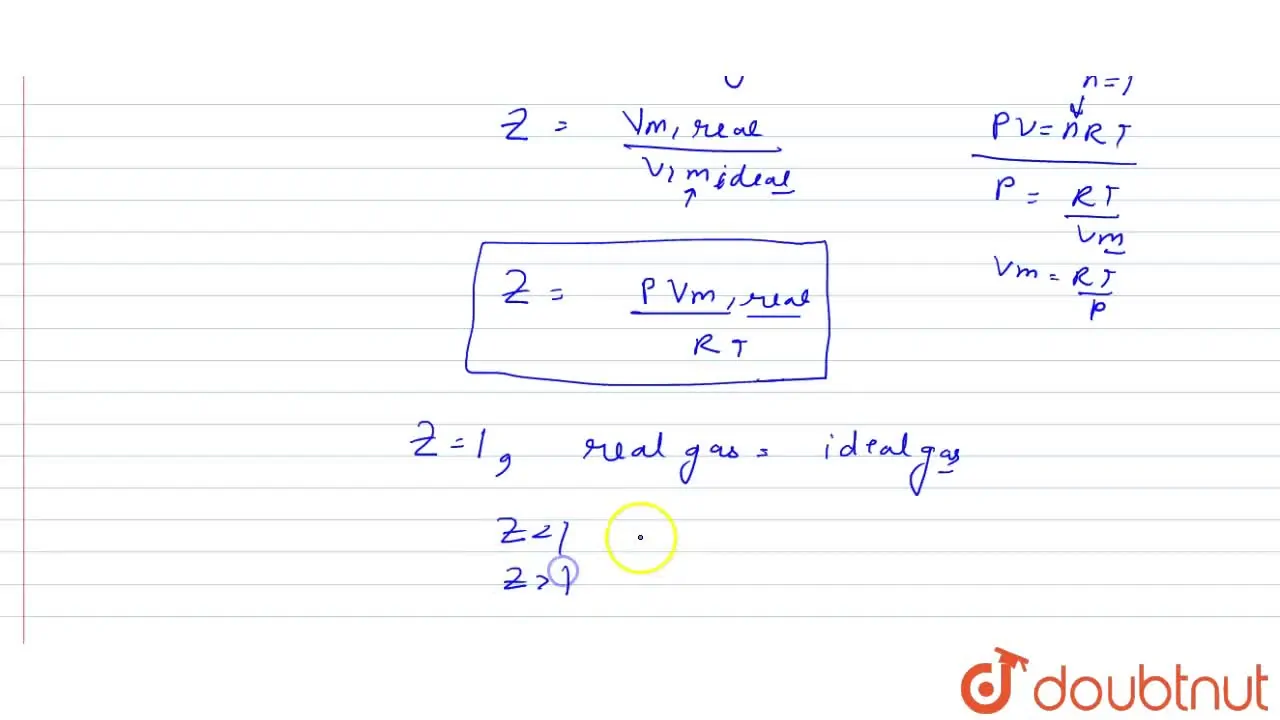
How is compressibility factor expressed in terms of molar volume of th