Solved] Why is the compressibility factor less than 1 at most
Answer to Why is the compressibility factor less than 1 at most conditions?
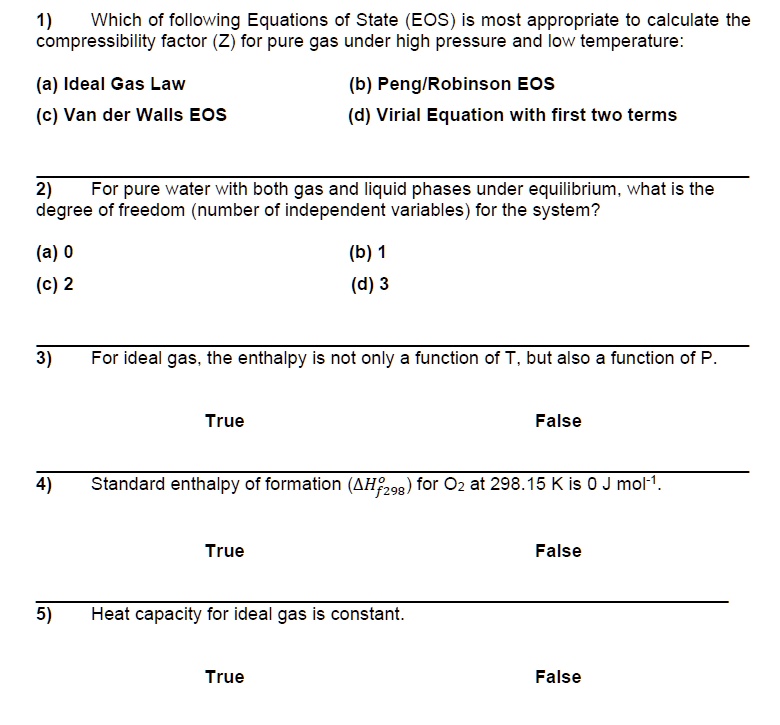
SOLVED: Just choose the right answer, no need for explanation

Compressibility Factor - an overview

Compressibility Factor of Gas Overview, Equation & Chart
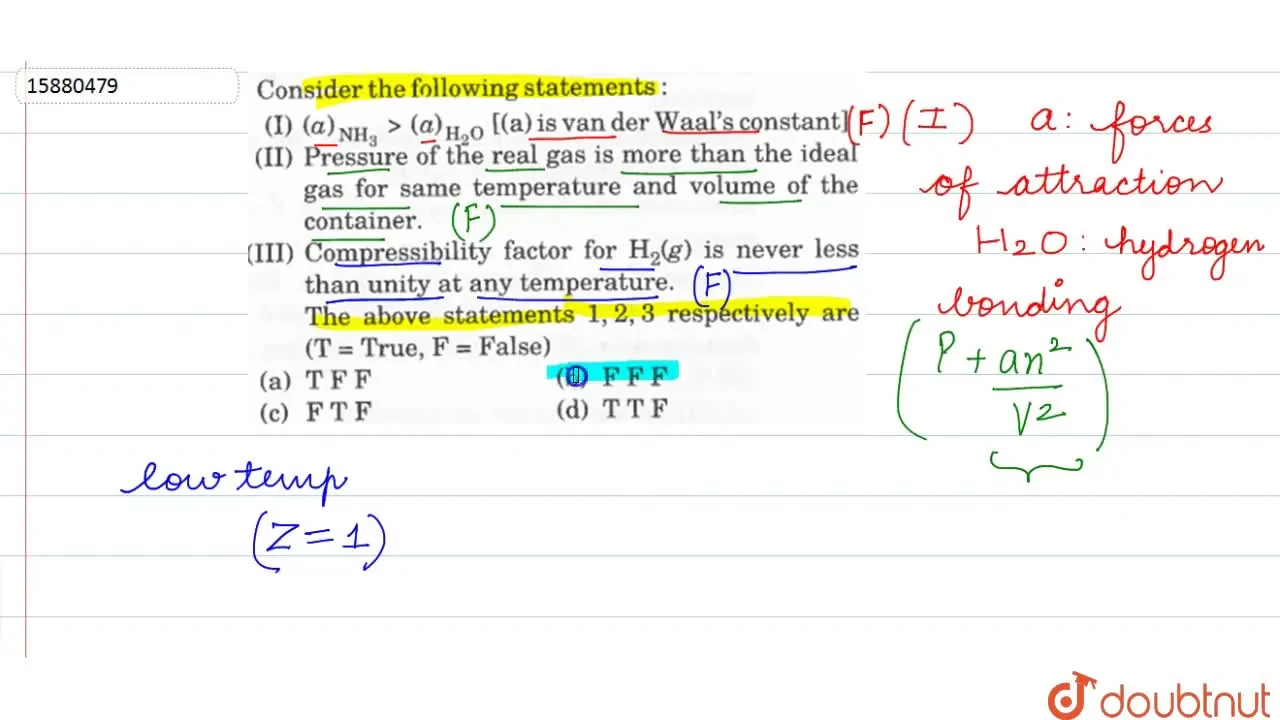
Consider the following statements: (I) (a)(NH(3))gt(a)+(H(2)O) [(a)

Van der Waals Equation - Derivation, Relation Between Ideal Gas

Acentric Factor - an overview

The compressibility of a gas is less than unity at STP. Therefore

The compressibility factor of a gas is less than 1 at STP. Its

Non-Ideal Gas Behavior Chemistry: Atoms First

gas laws - Compressible Factor - Chemistry Stack Exchange

Gas compressibility factor Z: Ideal gas vs Real gas

Statement-1. Compressibility factor of non-ideal gases is always

The compressibility factor (Z) for a gas is less than one.What does

3.2 Real gas and compressibility factor – Introduction to

Compressibility factor - Wikipedia









