
Establishing expiry date for clinical diagnostic reagents
Product shelf life is an essential product performance requirement that, along with other design requirements, is used to determine the safety and efficacy of a clinical diagnostic

Clinical Chemistry Reagent Alkaline Phosphatase For Diagnostic

Chemicals and Reagents Management in Quality Control Laboratory
National Action Plan to Build Australia's Diagnostic Technology
Industry Reacts to FDA Draft Guidance on Injectable Products

Update: UN Day Ministry of Health

An Easy Guide to Chemical Expiry Dates: Top 10 Lab Chemicals

Do COVID-19 tests still work after they expire? Here's how to tell

SEC Filing Akoya Biosciences, Inc.

ASTM D7160-16 Red - Standard Practice for Determination of

UNE EN ISO 23640:2015 In Vitro Diagnostic Medical Devices, 41% OFF

Latest News – iHealth Labs Inc
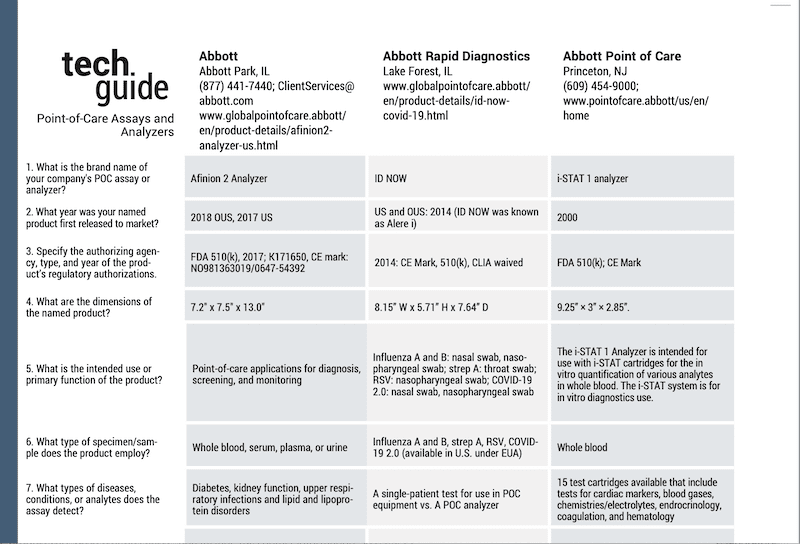
Clinical Lab Products

PDF) Practical considerations for navigating the regulatory

Wait list for COVID-19 test results balloons to 11,000 in Ontario









