
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
Under what conditions do you expect a real gas such as hydrogen gas to behave like an ideal gas? - Quora
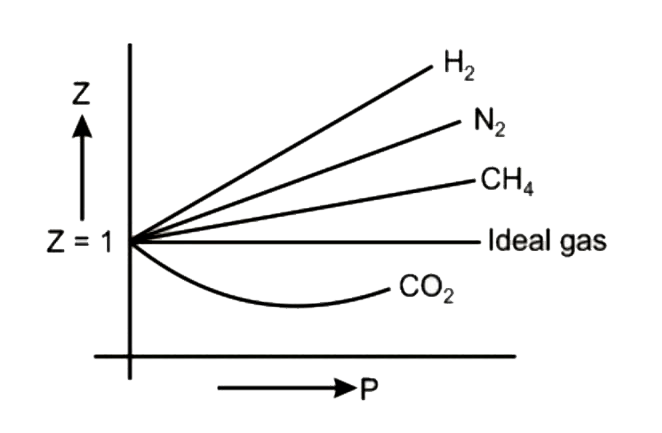
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure
1.5 Real Gases and the Virial Equation - Mail

Compressibility factor (Z = (P V/n R T)) is plotted against pressure What is the correct order of liquefiability of the gases shown in the
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora
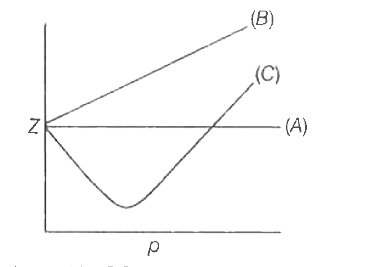
Telugu] The variation of compressibility factor (Z) with pressure (p
At which pressure methane gas becomes non ideal? - Quora

Non-Ideal Gas Behavior Chemistry: Atoms First

gas laws - Does the amount of a gas increase with pressure? - Chemistry Stack Exchange
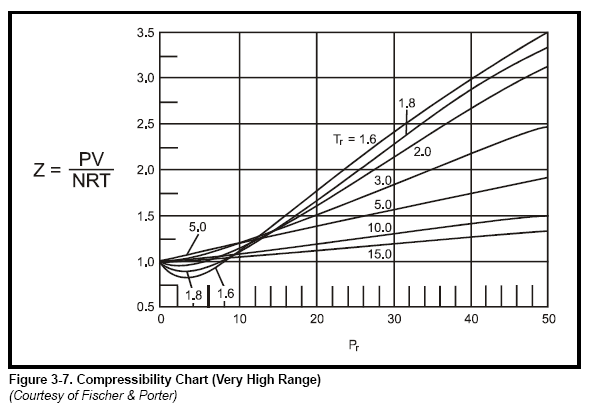
Chapter 3 - Physical Properties of Fluids: Gas Compressibility Factor

A real gas M behaves almost like an ideal gas. Graph 1 is obtained by plotting volume, V against temperature, T for x mol of gas M at pressure, P_1. a. Suggest
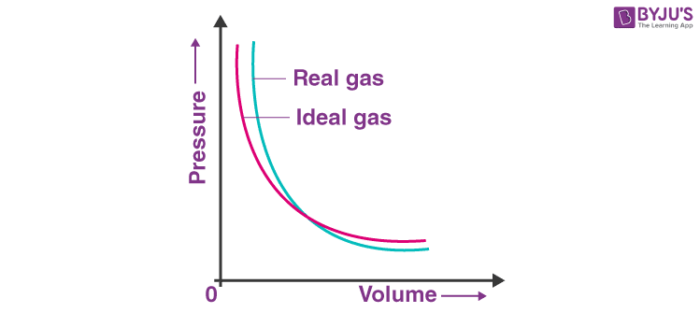
Deviation Of Real Gas From Ideal Gas Behavior









