Applications for Medical Device Investigational Testing Authorizations Guidance Document
4.9
(601)
Write Review
More
$ 26.99
In stock
Description
Applications for Medical Device Investigational Testing Authorizations Guidance Document

Common Problems to Avoid with IND Applications for New Drugs and Biologics - Criterion Edge

Health Canada medical device regulations

Guidance Document for the Completion of APHIS/CDC Form 5
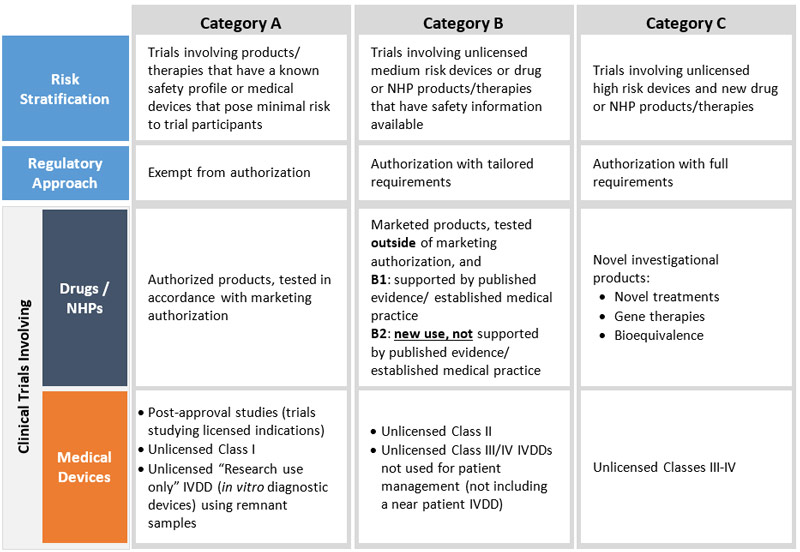
Clinical Trials Modernization: Consultation Paper

Current state of Health Canada regulation for cellular and gene

Permission To Conduct Clinical Investigation License, Medical

Class II - IV Medical Device Investigational Testing in Canada - Vantage BioTrials

Turkish TMMDA Guidance on Medical Device Withdrawals and Recalls

Pathways to a FDA Approved or Cleared Medical Device - StarFish Medical
Related products
You may also like


/i.s3.glbimg.com/v1/AUTH_59edd422c0c84a879bd37670ae4f538a/internal_photos/bs/2019/h/z/JiyEO7Q8mjBPJKaC3ANg/itamed-fachada.jpeg)