Crucial Steps for Singapore Medical Device Registration & HSA Approval
Discover the crucial steps for successful Singapore medical device registration and HSA approval. Operon Strategist offers expert guidance, classification insights, and comprehensive support. Contact Operon Strategist to learn more and navigate the regulatory landscape with confidence.

Credevo on LinkedIn: Drug Registration & Approval Process In Singapore
Medical Device Regulation: Importance and Examples in APAC

Kallol Sen on LinkedIn: #medicaldevices #invitrodiagnostics #productregistration

The Singapore Guidance on Software Medical Devices

Academic Report on Singapore HSA Class D and Australia TGA Class III Medical Device Regulatory Overview & Strategy

HSA Guidance on Medical Device Product Registration: Additional Aspects
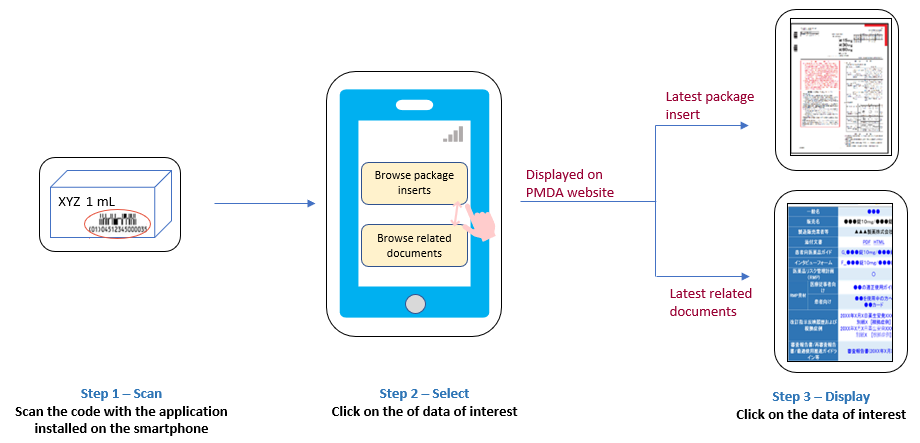
E-labeling and digital transformation in healthcare

Overview of medical device regulations in Canada, Journal
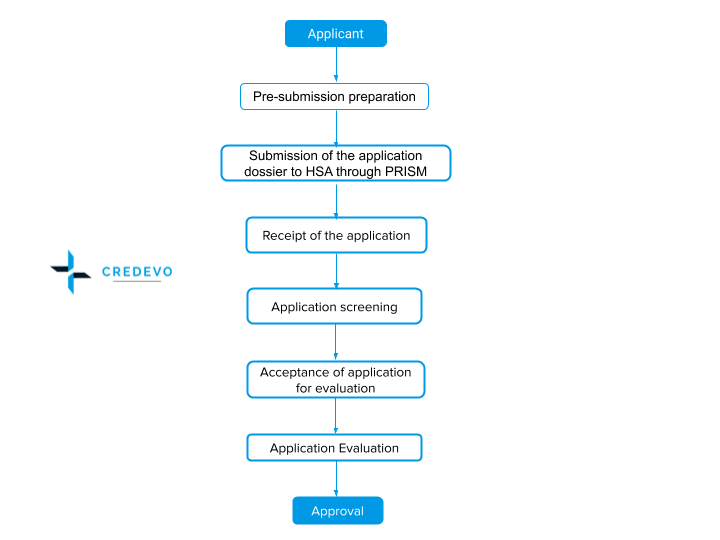
Generic Drug Registration Process In Singapore

Is it possible to obtain approval for entry in the ARTG based on overseas approvals?

Device Firms In Non-EU Markets Using The CE Mark Should Expect Some MDR Disruption After May :: Medtech Insight