The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect
The compression factor (compressibility factor) for one mole of a van der Waals
A gas described by van der Waals' equation (a) behaves similar to an ideal gas molar volumes - Sarthaks eConnect

Solved APPENDIX Problem 1: Molar Volume and Compressibility
The compression factor (compressibility factor) for one mole of a van der Waals' gas - Sarthaks eConnect

The compression factor (compressibility factor) one mole of a van der Waals' gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is negligible
The compressibility factor for a real gas at high pressure is - Sarthaks eConnect
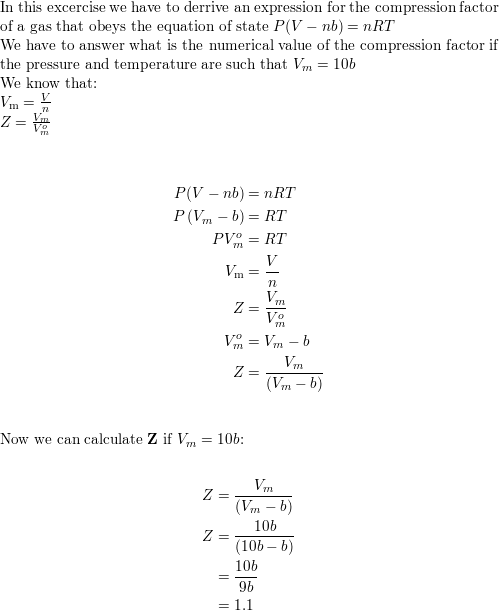
Derive an expression for the compression factor of a gas tha

Solved We begin by showing that the compressibility factor

The compressibility factor 1 mole of vanderwaal gas 0^{o}C, and 100 atm pressure is found to be 0.5, then calculate the vander Waals constant a. Assuming: that the volume of gas molecule
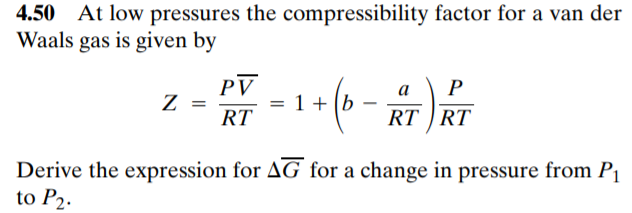
Solved 4.50 At low pressures the compressibility factor for

States Of Matter Notes: Class 11, JEE, NEET, AIIMS

The compressiblity factor a gas obeying van der Waals' equation of state is given by V V-b RTV (2) a ✓ RTV V-b V-b RTV (3) Va (4) RTV V-6