For a given gas, a graph is shown between compressibility factor
By the graph, at T2, the gas does not deviate from ideal gas behaviour at low pressure (Z = 1).Hence, T2 = TBT2 = TB (Boyle's temperature)T1 > TB (Here, Z > 1)This implies that T3 B and TB > TC(Here, Z So, T1 and T2 must be above TC.

For one mole of a real gas, curves are plotted under different conditions the same temperature as shown in diagram: slope = 102 2463 C (In low pressure region) RT slope =

REAL GASES, DEVIATION FROM IDEAL GAS BEHAVIOUR

Compressibility Factor - an overview

PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

The curve of pressure volume PV against pressure P of the gas at a particular temperature is as shown, according to the graph which of the following is /are incorrect in the

Compressibility factor (z): real gases deviate from ideal behav-Turito

Compressibility factor (Z) is plotted against pressure at different te

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT
The effect of Pressure on Temperature-Compressibility Factor diagram

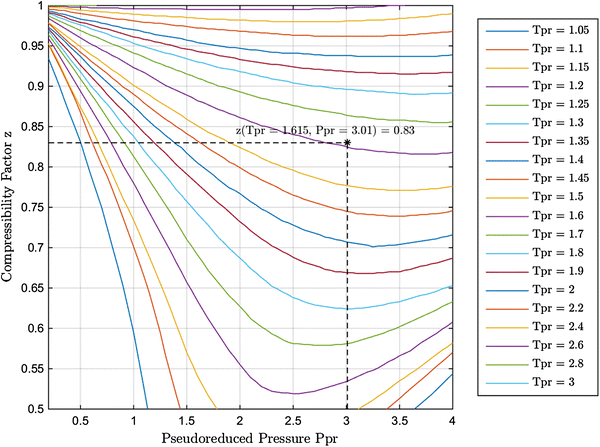
Real-gas z-factor, as attributed to Standing and Katz, 9 plotted

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson
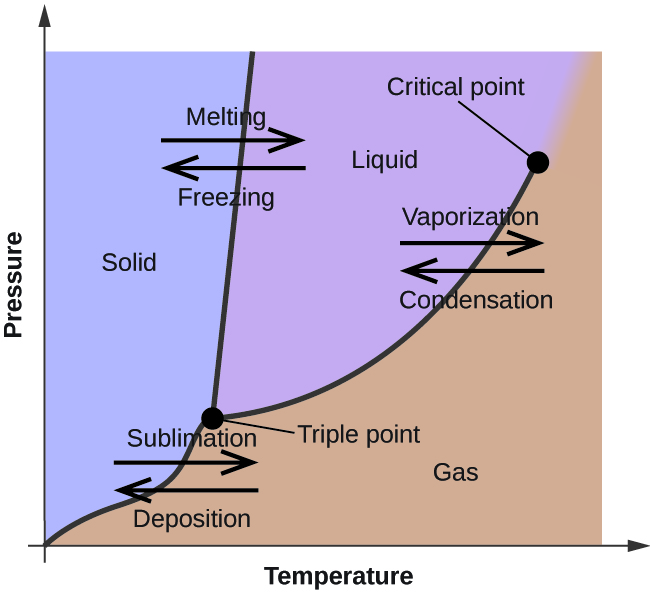
Phase Diagrams Chemistry for Majors

In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2