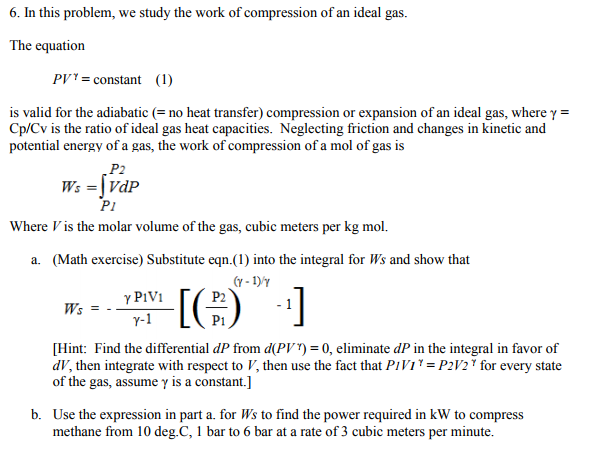
Solved ion of an ideal gas. The equation PI = constant (1)
Consider Hydrogen ion in a box having one dimension, then find electron probability distribution?

Use the van der Waals equation and the ideal gas equation to calc

Solved ion of an ideal gas. The equation PI = constant (1)

TPSS 640 - Chapter 2

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law
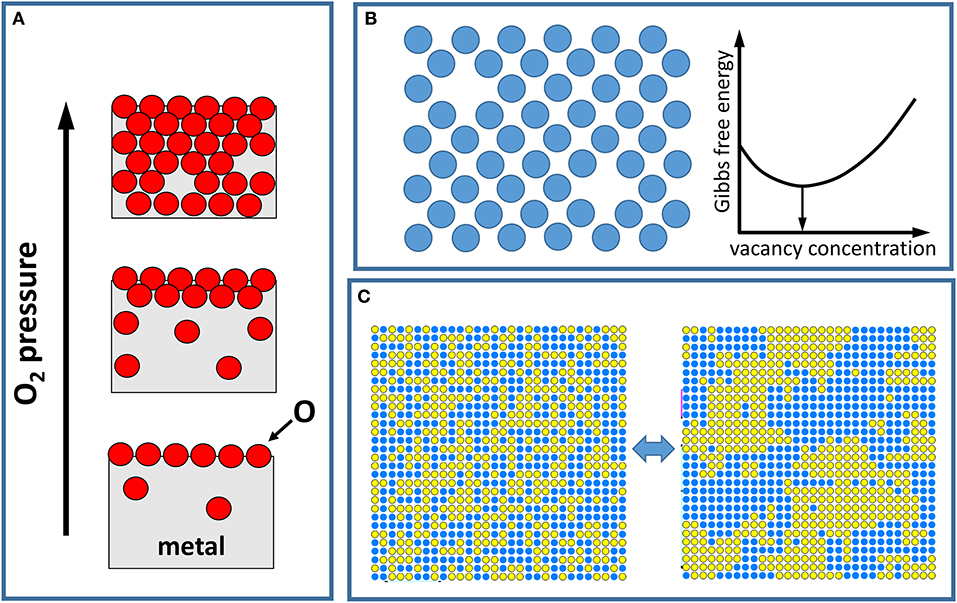
Frontiers First-Principles Atomistic Thermodynamics and Configurational Entropy
How is the value of k (Boyle's law constant) different for a different amount of the same gas? - Quora

Use the van der Waals equation and the ideal gas equation to calc

Ideal Monatomic Gas - an overview
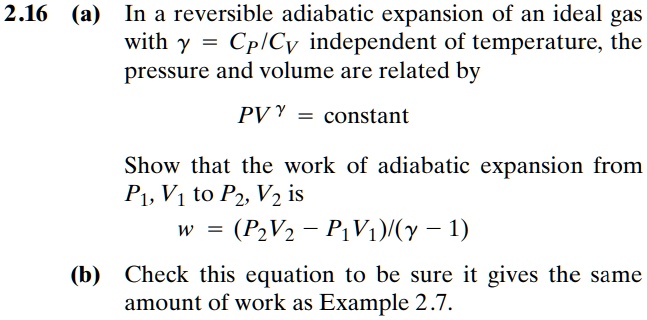
SOLVED: 2.16 (a) In a reversible adiabatic expansion of an ideal gas with y = Cp/Cv independent of temperature, the pressure and volume are related by PV = constant. Show that the
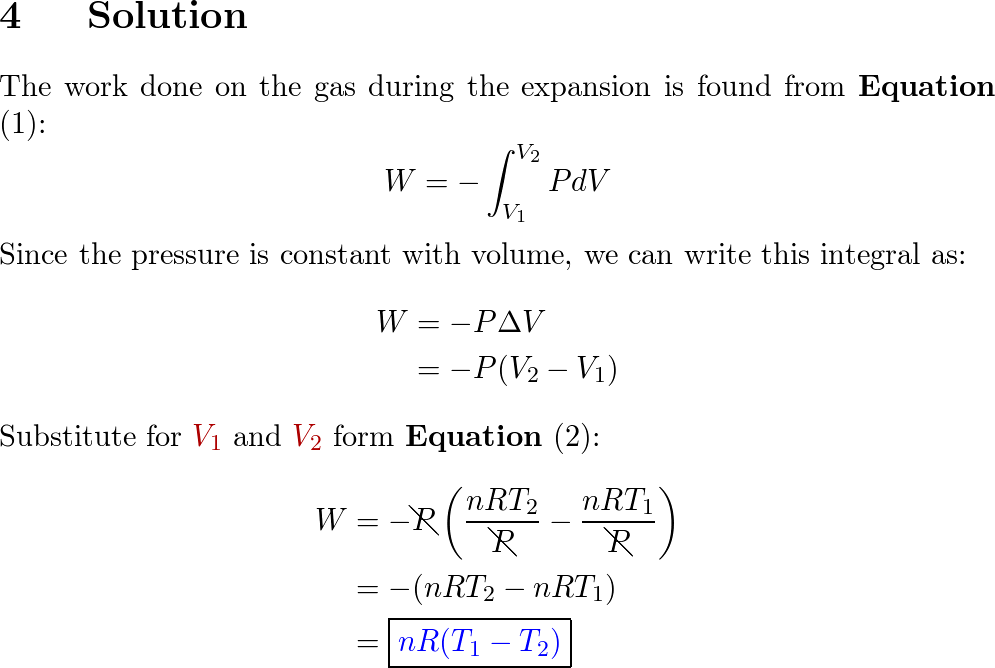
An ideal gas is enclosed in a cylinder that has a movable pi









