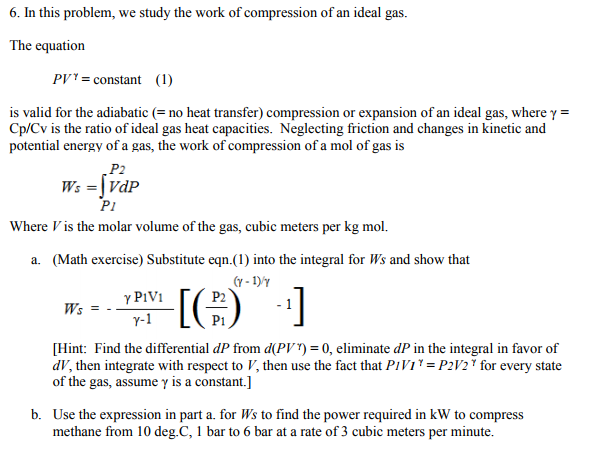
Solved ion of an ideal gas. The equation PI = constant (1
4.9
(701)
Write Review
More
$ 7.50
In stock
Description
Processes, Free Full-Text
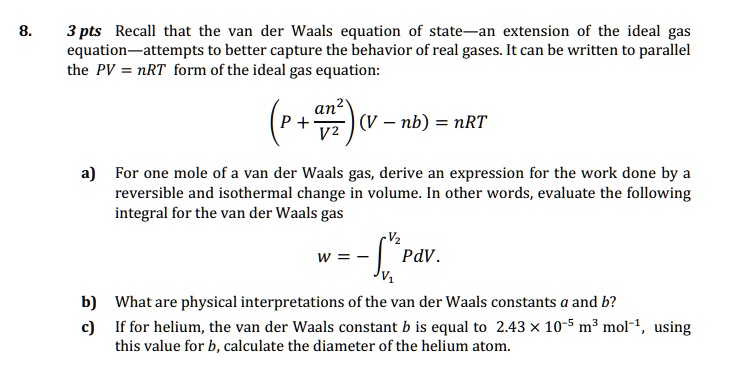
SOLVED: Recall that the van der Waals equation of state is an

Derivation of limiting ion mobility equation based on the
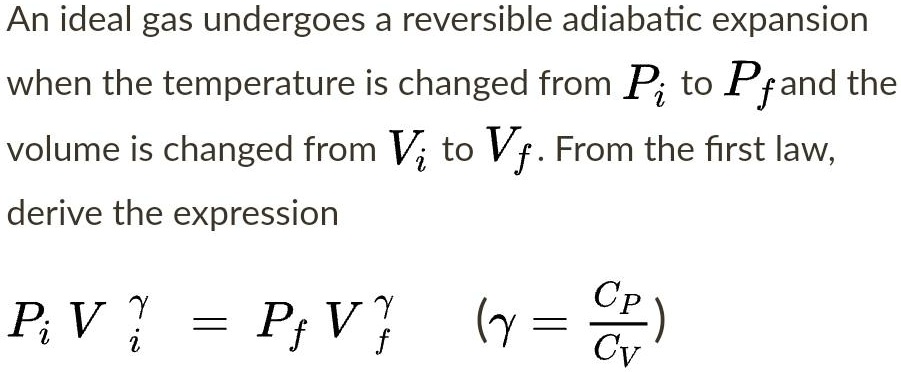
SOLVED: An ideal gas undergoes a reversible adiabatic expansion

Activity Coefficient: Formula, Equation & Solved Questions
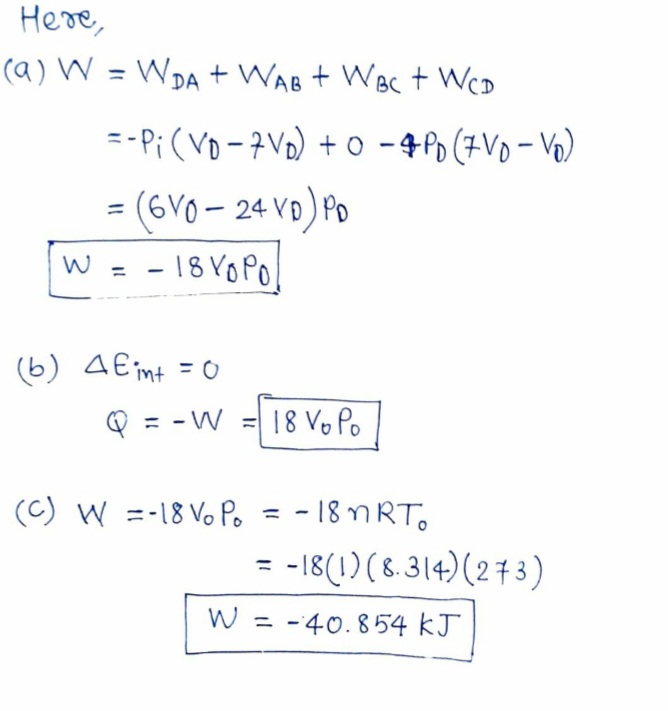
Answered: An ideal gas initially at pressure P0,…

Osmotic pressure - Wikipedia
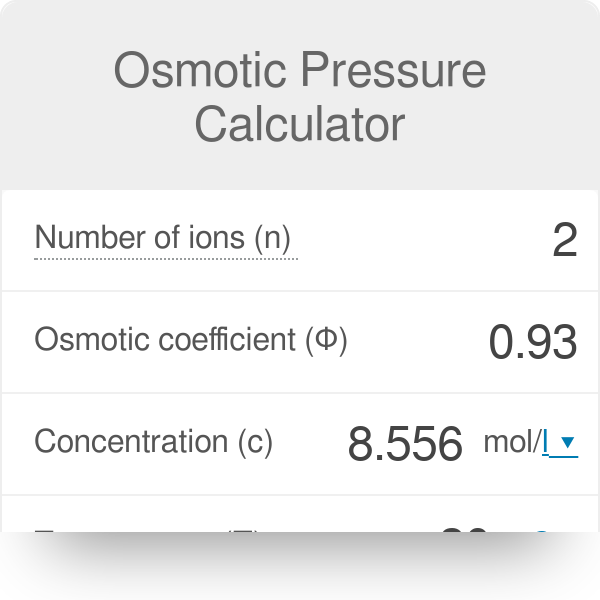
Osmotic Pressure Calculator

Chemistry - Unit 3 - Joseph Flashcards
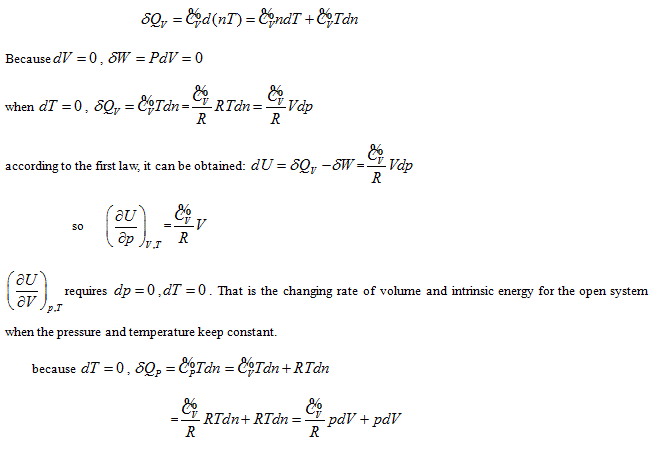
State and equal-intrinsic energy curved surfaces of ideal gas

Ideal Monatomic Gas - an overview
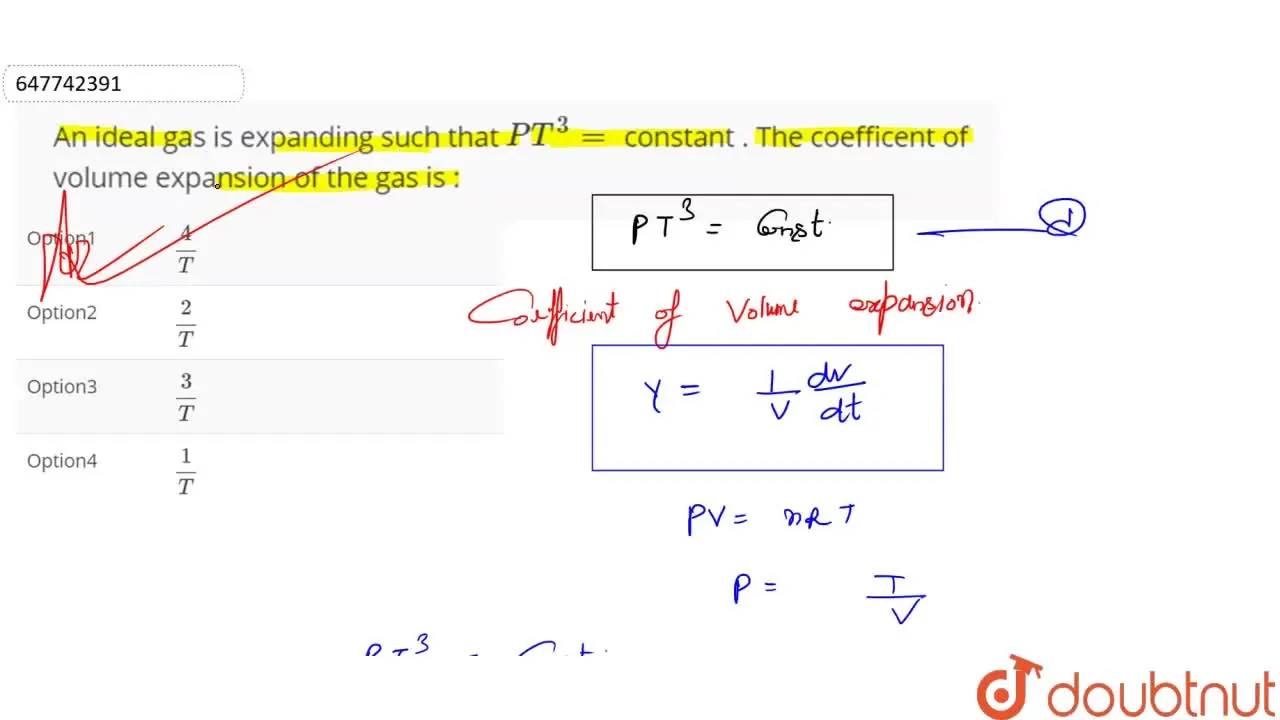
An ideal gas is expanding such that PT^(3)= constant . The coefficent
Related products
You may also like