
1) 2 23 g ethanol is dissolved in 36 g water. Find mole fraction
Click here:point_up_2:to get an answer to your question :writing_hand:1 223 g ethanol is dissolved in 36 g water find mole fraction of ethanol2
Click here👆to get an answer to your question ✍️ -1- 2 23 g ethanol is dissolved in 36 g water- Find mole fraction of ethanol -2- 0-5 -3- 0-2 -4- 0-8 TIN

Telugu] 0.46g of Ethanol is dissolved in 1000 g of H2O. What is the m
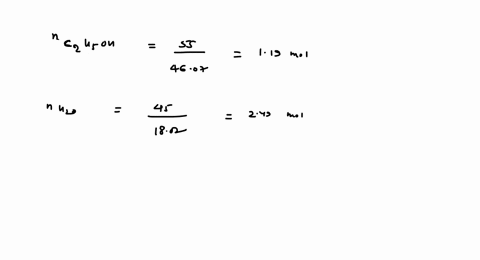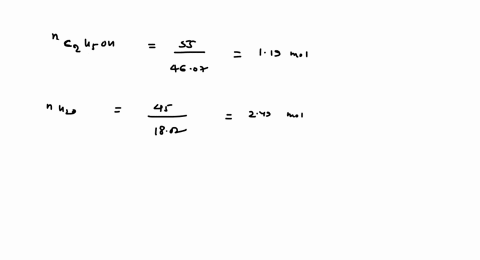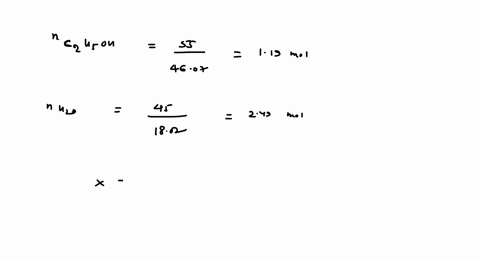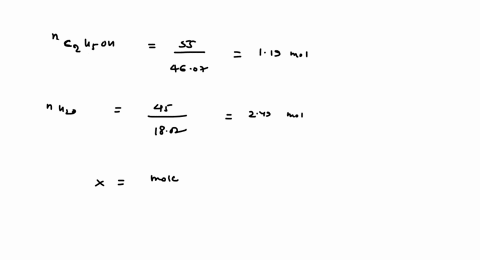
SOLVED: 23 gram ethanol is dissolved in 36 gram water. Find mole

SOLVED: 10 g ethanol dissolved oxygen in 50g of water . calculate

Calculate mole fraction of ethyl alcohol and water in a solution containing 46 g ethyl and 36g w

Calculate the molality of ethanol solution in which the mole

Ethanol - Wikipedia

Calculate the molality of a solution of Ethanol (C_2H_5OH) in

23 g ethanol is dissolved in 36 g water. Find mole fraction of
23 g ethanol is dissolved in 36 g water. Find mole fraction of

A mixture has 18 g water and 414 g ethanol . The mole fraction of

23 g ethanol is dissolved in 36 g water. Find mole fraction of
In a certain solution of ethanol and water, the mole of fraction
What are the mole fractions of ethyl alcohol (C2H5OH) and water
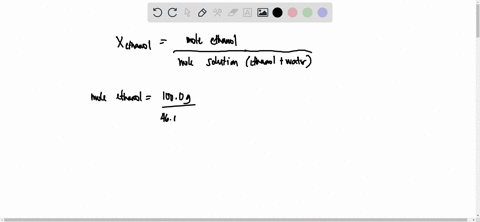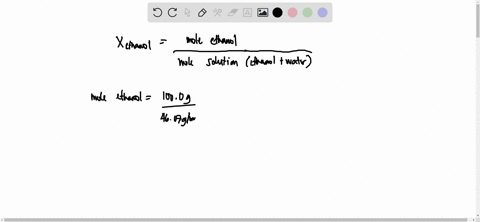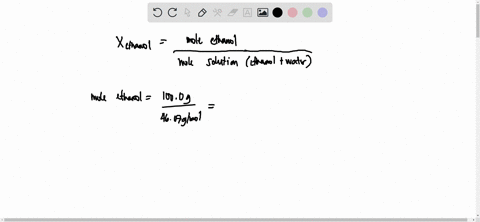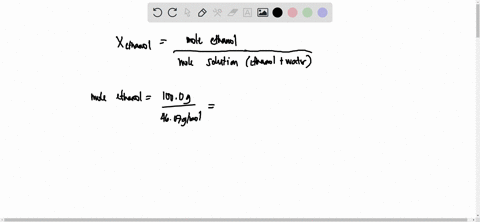
SOLVED: 23 gram ethanol is dissolved in 36 gram water. Find mole









