Color change is only device modification. Is a new 510k required? - Medical Device Academy
This article explains the process for determining if a color change and other material changes require a new 510k prior to implementing the change.
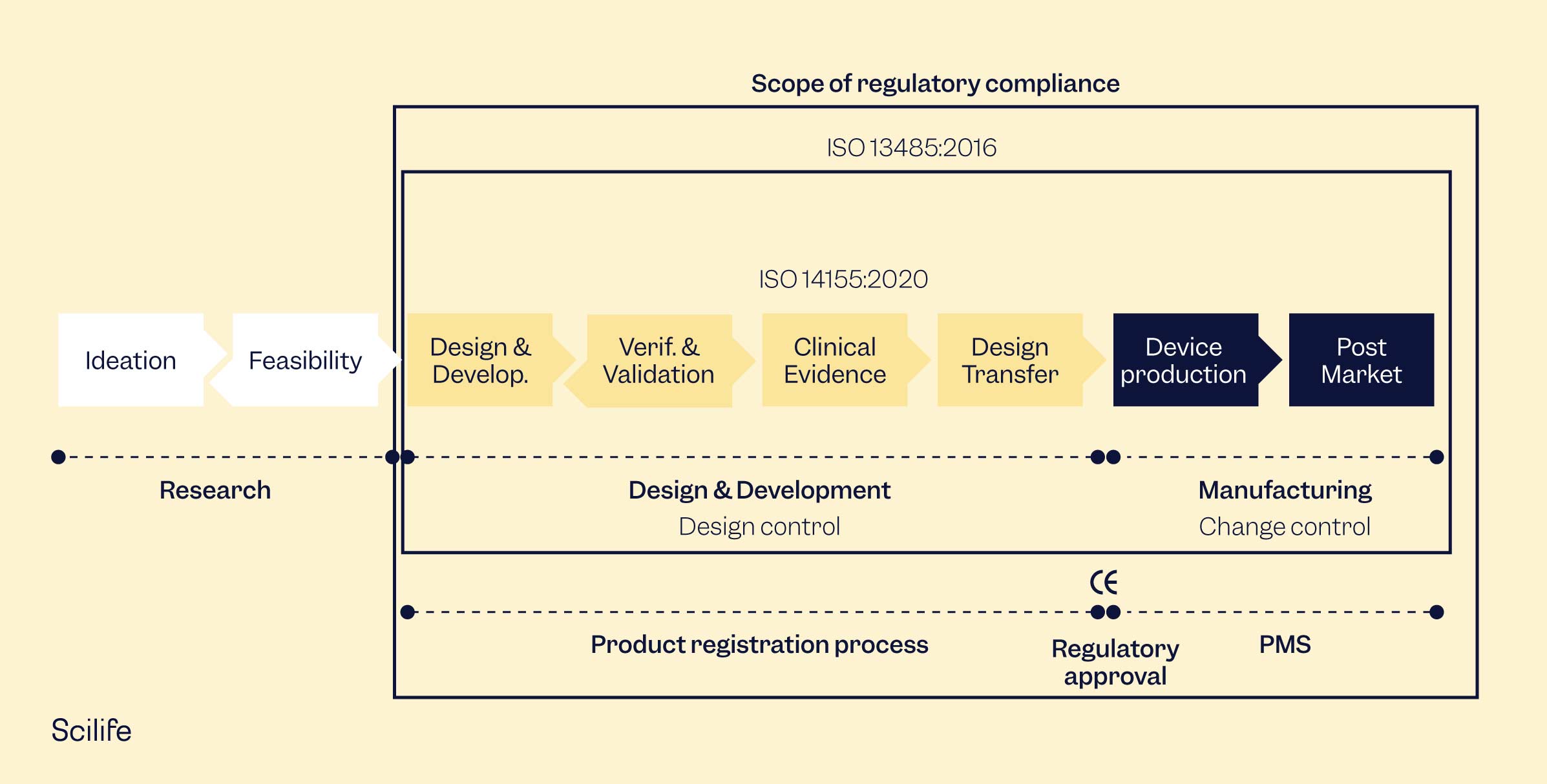
A Guide to Bringing a Medical Device to Market

Case Study: FDA Regulatory Responsibilities for Color Additives
%20device%20software%20hardware.png?width=606&height=522&name=510(k)%20device%20software%20hardware.png)
Everything you need to know about the FDA 510(k) submission

Tens Unit Plus 24 Rechargeable Electronic Pulse Massager Machine Multi Mode Device with All Accessories [New Model] : Health & Household
Does Your Device Modification Qualify For A Special 510(k)?

25 FAQs (& Answers) about ISO 15223-1:2021 Fourth Edition
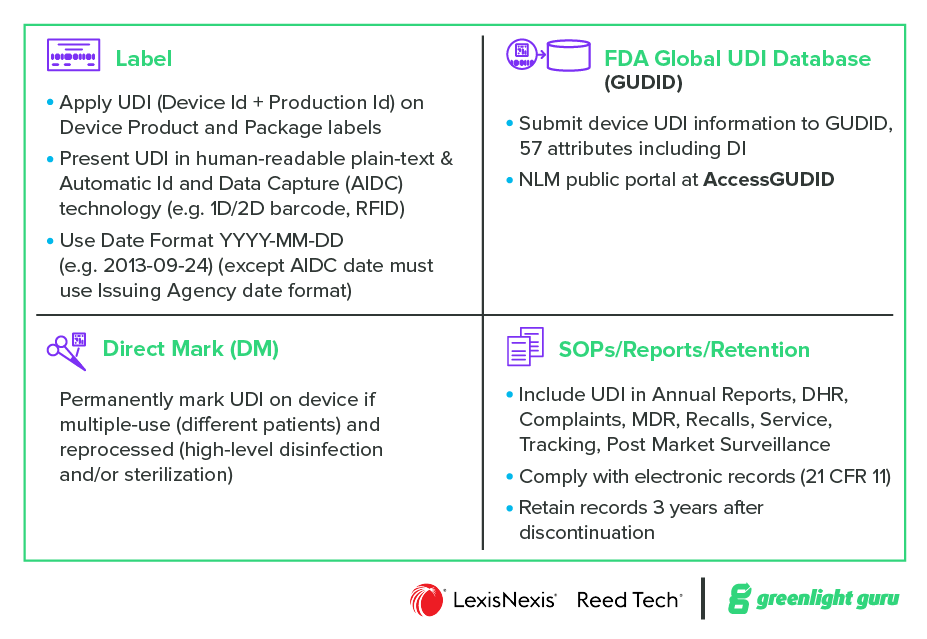
Ultimate Guide to UDI for Medical Devices
.png)
Definitive Guide to Change Management for Medical Devices

A decades-long fight over an electric shock treatment led to an
Understanding the New FDA Guidance on Changes to a 510(k)

FDA

FDA 510(k) Database

The Top 10 Most-Cited Clauses In FDA FY2021 Medical Device Inspections

Ultimate Guide to QA & RA in Medical Device 3D Printing