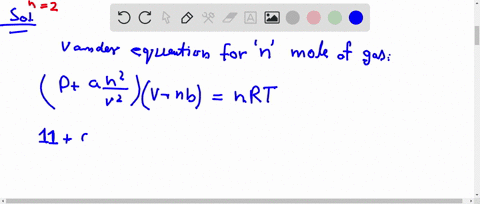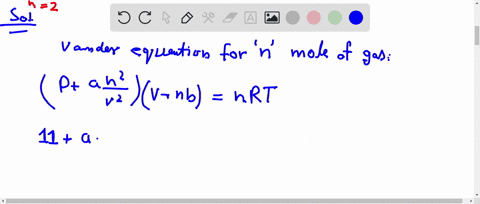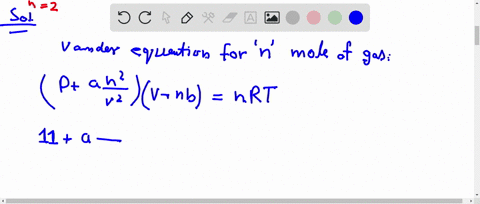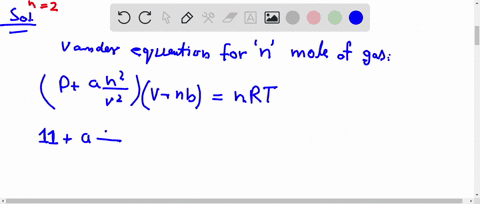
What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

physical chemistry - Why do some gases have lower value of Z for a particular pressure? - Chemistry Stack Exchange

Effects of Graphene Oxide Nanosheets and Al2O3 Nanoparticles on CO2 Uptake in Semi‐clathrate Hydrates - Hassan - 2021 - Chemical Engineering & Technology - Wiley Online Library
⏩SOLVED:The compression factor (compressibility factor) for one mole…

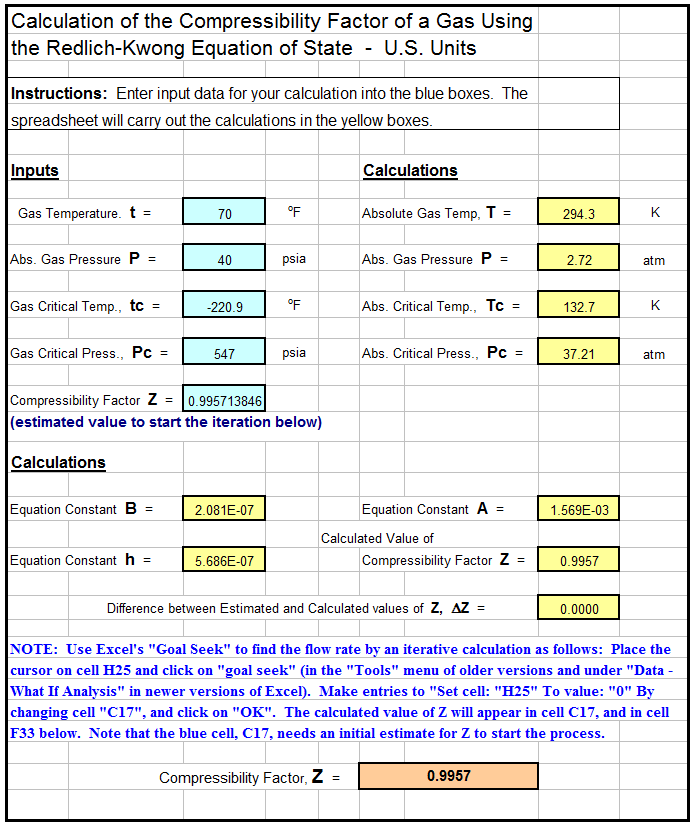
Determine Compressibility of Gases

McMurry and Fay On-Line Chapters

Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of

Sheet - 01 - Real Gas, PDF, Gases
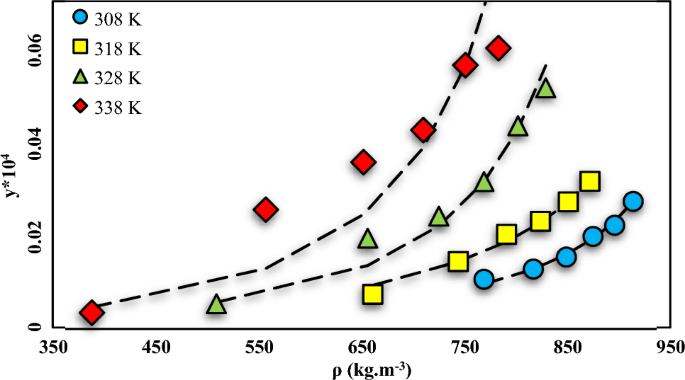
A machine learning approach for thermodynamic modeling of the statically measured solubility of nilotinib hydrochloride monohydrate (anti-cancer drug) in supercritical CO2

Solved 9 Compression factor Z Use the van-der-Waals equation

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

Solved The van der Waals equation of state can be used to