/cloudfront-us-east-1.images.arcpublishing.com/pmn/EMEDJI4AQVBZJNJHKLTY2ZSPJY.jpg)
FDA has new mammogram guidelines for dense breast disclosure. What do the rules mean for Pennsylvania residents?
In Pennsylvania, senators unanimously voted in favor of a bill to fund genetic testing to women at higher risk of breast cancer.

FDA Will Require Dense Breast Disclosure at Mammogram Clinics - The New York Times

Rad Tech CE, ASRT, ARRT® CE, Category A Credits
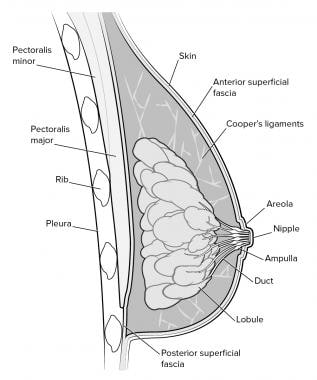
Mammography in Breast Cancer: Background, X-ray Mammography, Ultrasound

Breast Density and Breast Cancer Risk: A Practical Review - ScienceDirect

Rad Tech CE, ASRT, ARRT® CE, Category A Credits

The FDA's rule change requiring providers to inform women about breast density could lead to a flurry of questions

Breast density: why all the fuss? - ScienceDirect

FDA to require mammogram reports include breast density information

FDA to Implement New Mammogram Regulations to Support Women with Dense Breasts