The entropy change for the conversion of 36 g water to vapour at
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is -Enthalpy of vaporization for water is 40-63 kJ mol-1

If water vapor is assumed to be a perfect gas, molar enthalpy change for vaporization of 1 mol of

The concept of dynamic evaporation enabled by reconfigurable Fe3O4@G
Solubility - Wikipedia

Calculate the entropy change for vaporization of `1mol` of liquid water to stem at `100^(@)
Sustainability, Free Full-Text

Enthalpy of vaporization - Wikipedia

Calculate the entropy change of n-hexane when 1 mole of it evaporates
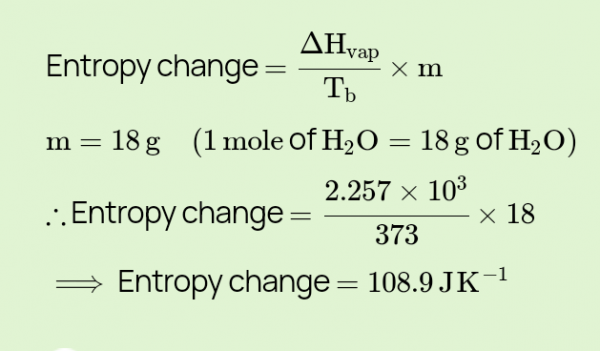
calculate the change in entropy for the conversion of one mole of liquid water to - Myschool

66. The entropy change for the conversion of 36 g of water to vapour at 100°C (Normal boiling point) is
6. The entropy change for the following reversible process 1 mole H2O(liquid 1atm 100^° c) 1 mole H2(gas,1atm,100^° c)(Δ Hvap=40850j/m) a.+109.52j/k/m b. 109.52j/k/m c.0.00jole/k/mole d.+6.084j/k/m

Energies, Free Full-Text

What is the entropy change (in JK^(-1)mol^(-1)) when one mole of ice i
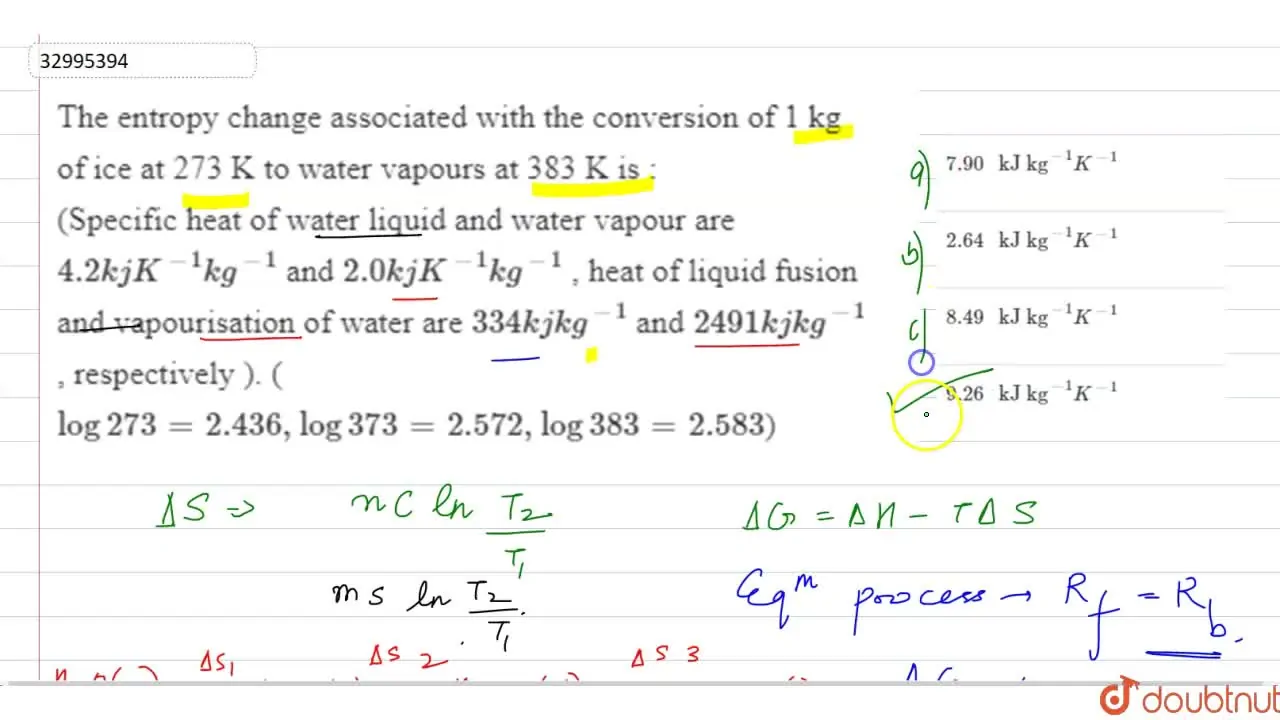
The entropy change associated with the conversion of 1 kg of ice at 27









