
Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be
Click here:point_up_2:to get an answer to your question :writing_hand:compressibility factor z of a gas is given as z frac pv nrt
Click here👆to get an answer to your question ✍️ Compressibility factor- Z of a gas is given as Z- frac - pV - nRT - -i- What is the value of Z an ideal gas-ii- For real gas what will be the effect on value of Z above Boyle temperature

Compressibility Factor of Gas Overview, Equation & Chart

1 CHAPTER 6 PROPERTIES OF GASES 6.1 The Ideal Gas

Energies, Free Full-Text
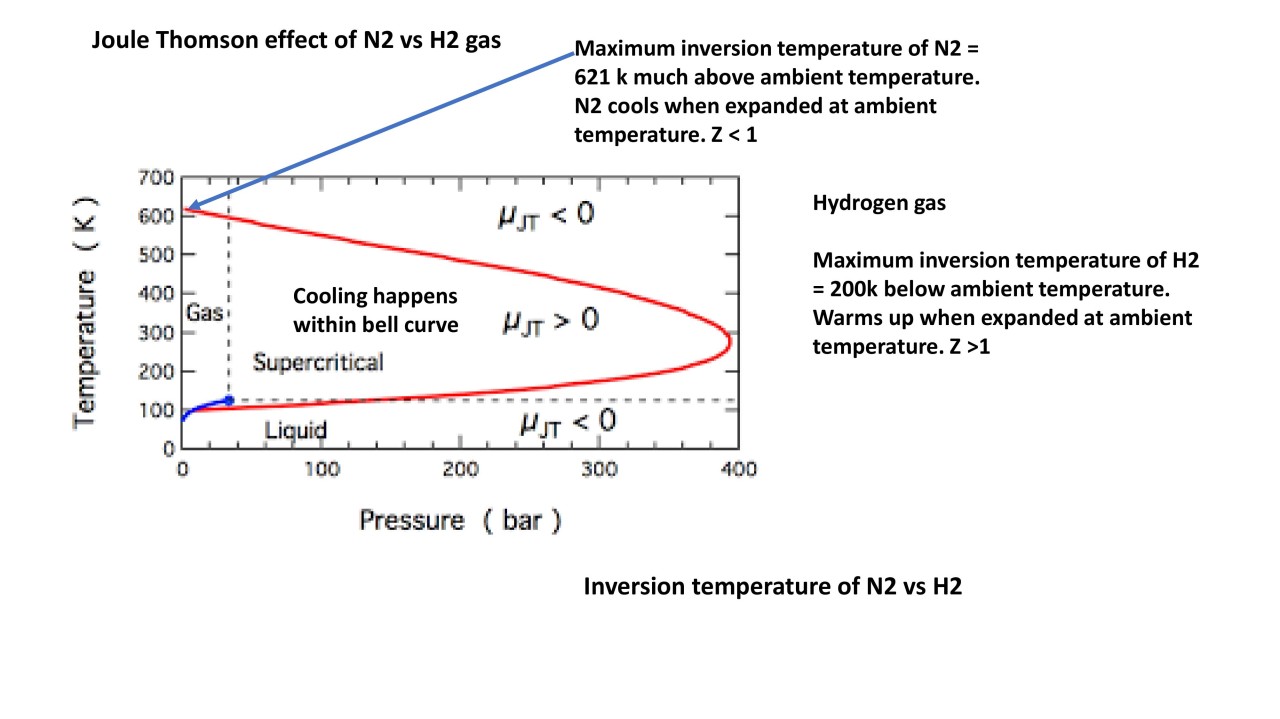
Joule Thomson effect [JT]: A short review

physical chemistry - Why do some gases have lower value of Z for a

Energies, Free Full-Text

Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i

Non-Ideal Gas Behavior Chemistry: Atoms First

PChem Quiz #1 Flashcards

Ideal Gas Equation - an overview

PPT - The Ideal Gas PowerPoint Presentation, free download - ID









