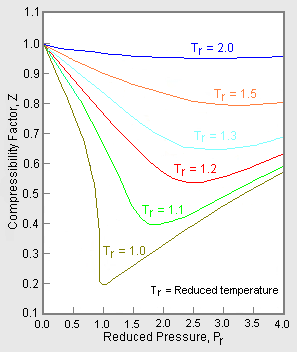
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas
What is the value of compressibility factor in terms of vander waal cons-an t at different conditions of pressure and volume-Why is Z-1 for H2 and He gas
Which gas shows the maximum deviation from ideal gas, CO2 or NH3? Why? - Quora

Properties of Gas Manik

Compressibility factor vs. number of cycles for a typical isobar.

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

A real gas M behaves almost like an ideal gas. Graph 1 is obtained by plotting volume, V against temperature, T for x mol of gas M at pressure, P_1. a. Suggest

1.7: Connecting the van der Waals and the viral equations- the Boyle temperature - Chemistry LibreTexts

Solved Real gas effects can be expressed as departures from

Real Gases Introductory Chemistry

Compressibility factor (gases) - Knowino